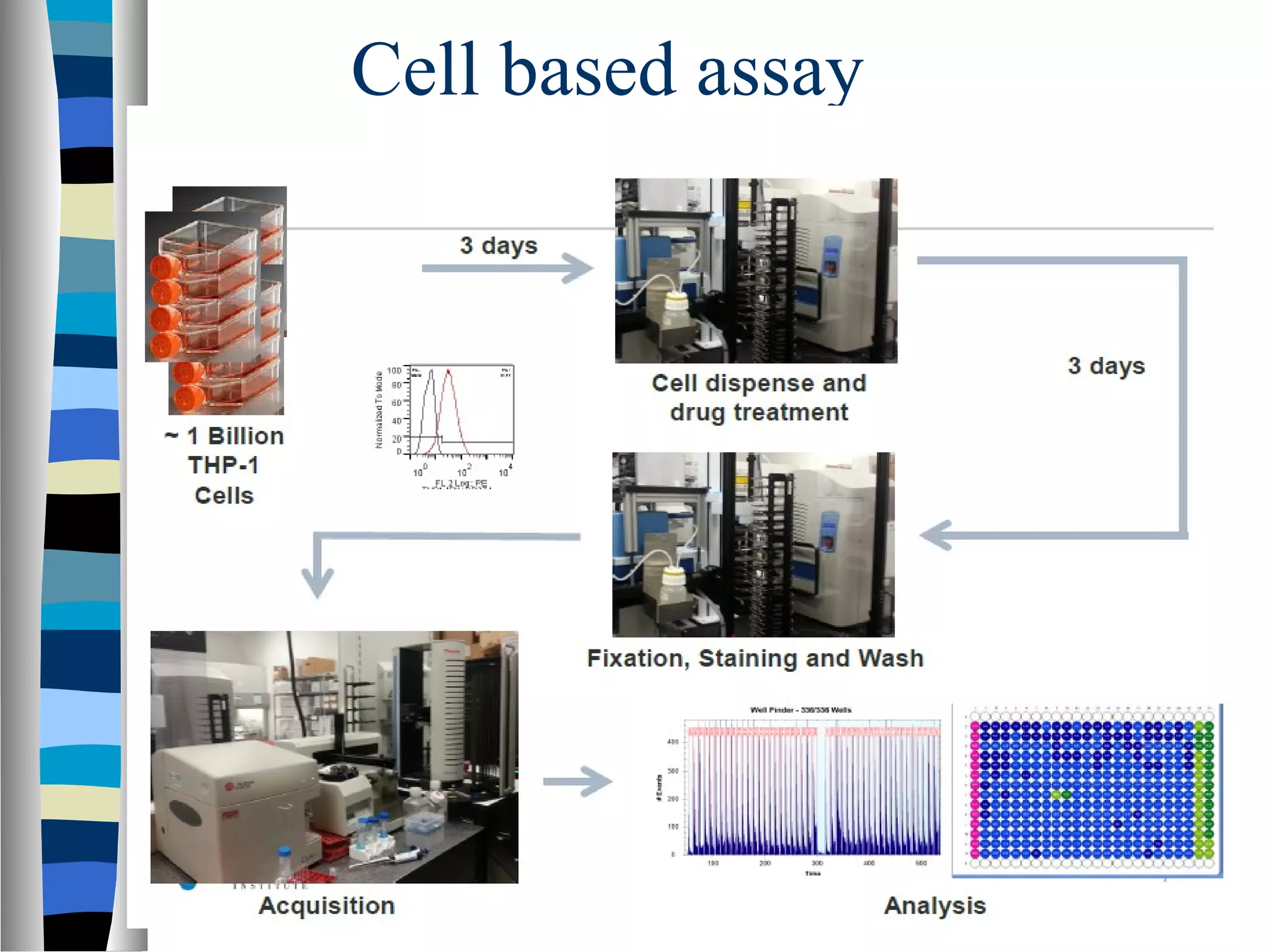
High Throughput Screening (HTS) involves the automated screening of large libraries of chemical compounds against biological targets at a rapid pace. HTS uses robotics, miniaturization, and automation to screen 50,000-100,000 compounds per week. The goals of HTS are to identify compounds that selectively bind and modulate biological targets of interest. Hits identified through primary screening are then evaluated further through secondary assays, SAR analysis, and in vitro/in vivo studies. HTS accelerates drug discovery by allowing for the rapid evaluation of large compound libraries.


![Goals
1. Identify a molecular structure that:
• Selectively binds to and modulates the
activity of a biological target (e.g., a
protein) of interest [target-based]
• Selectively induces a desired
phenotype in a cell population or
organism of interest [phenotype-based]](https://image.slidesharecdn.com/f81cc029-69d6-487f-8058-63ed9f412260-160315075547/75/HTS-3-2048.jpg)