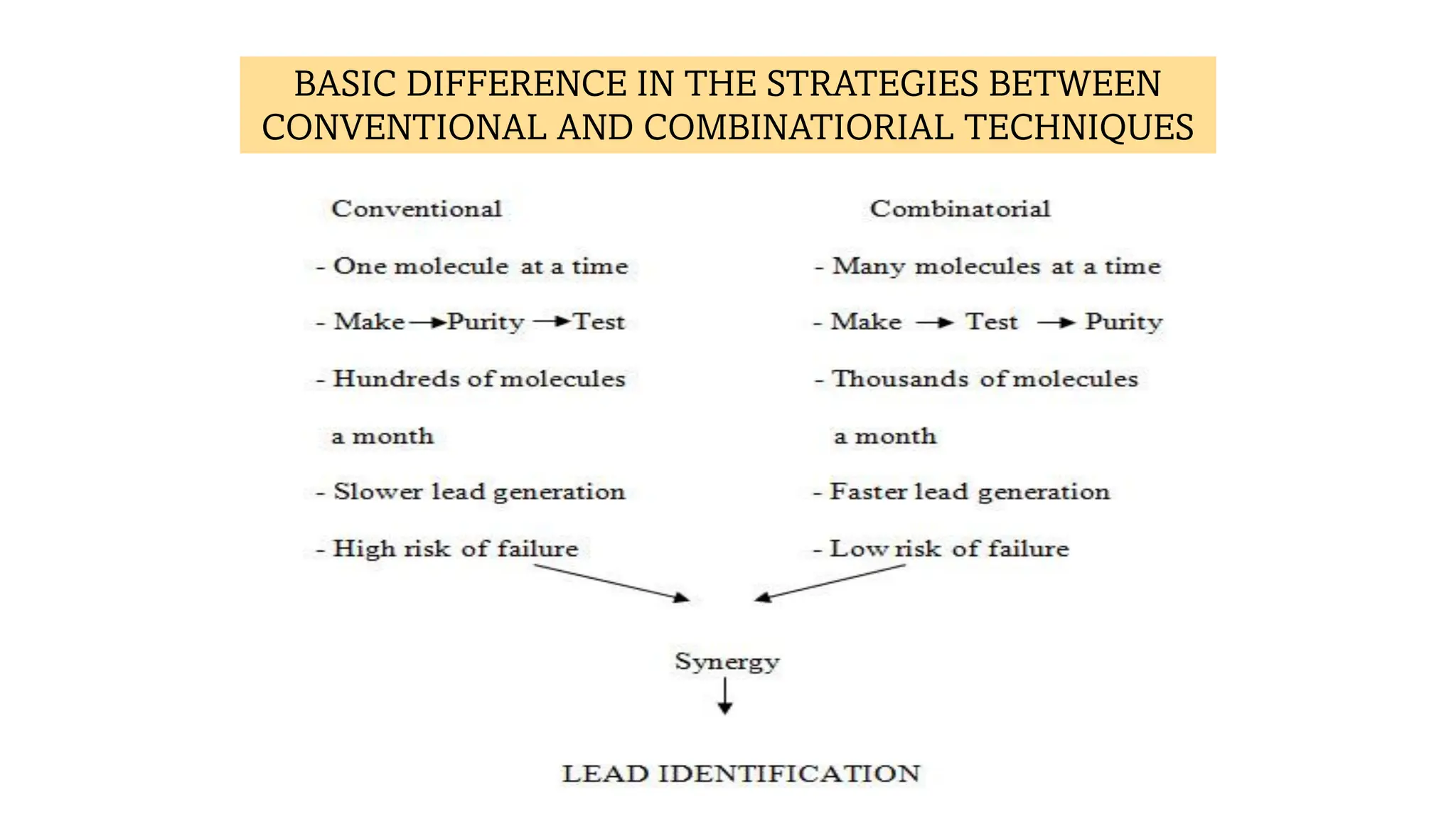
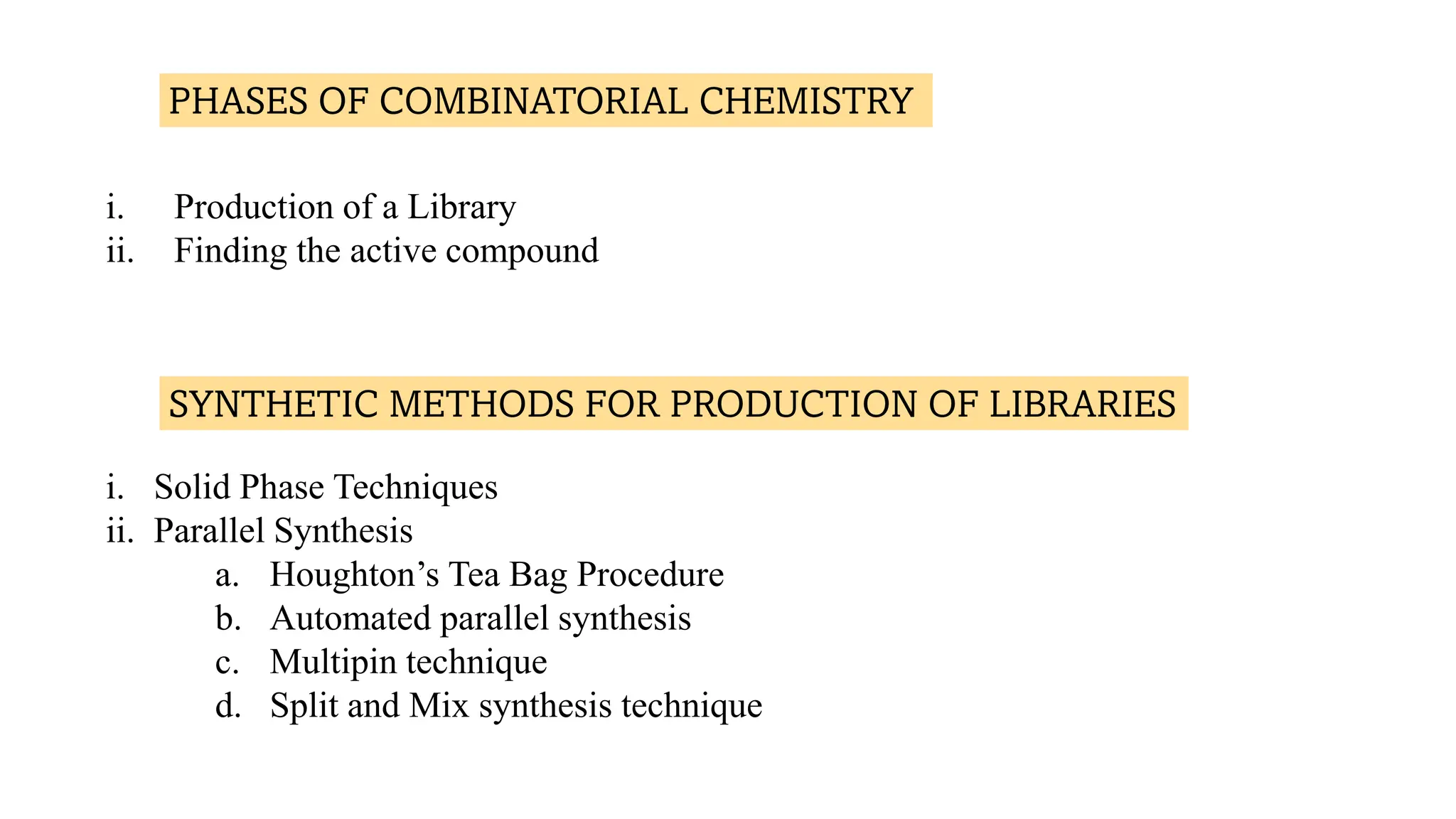
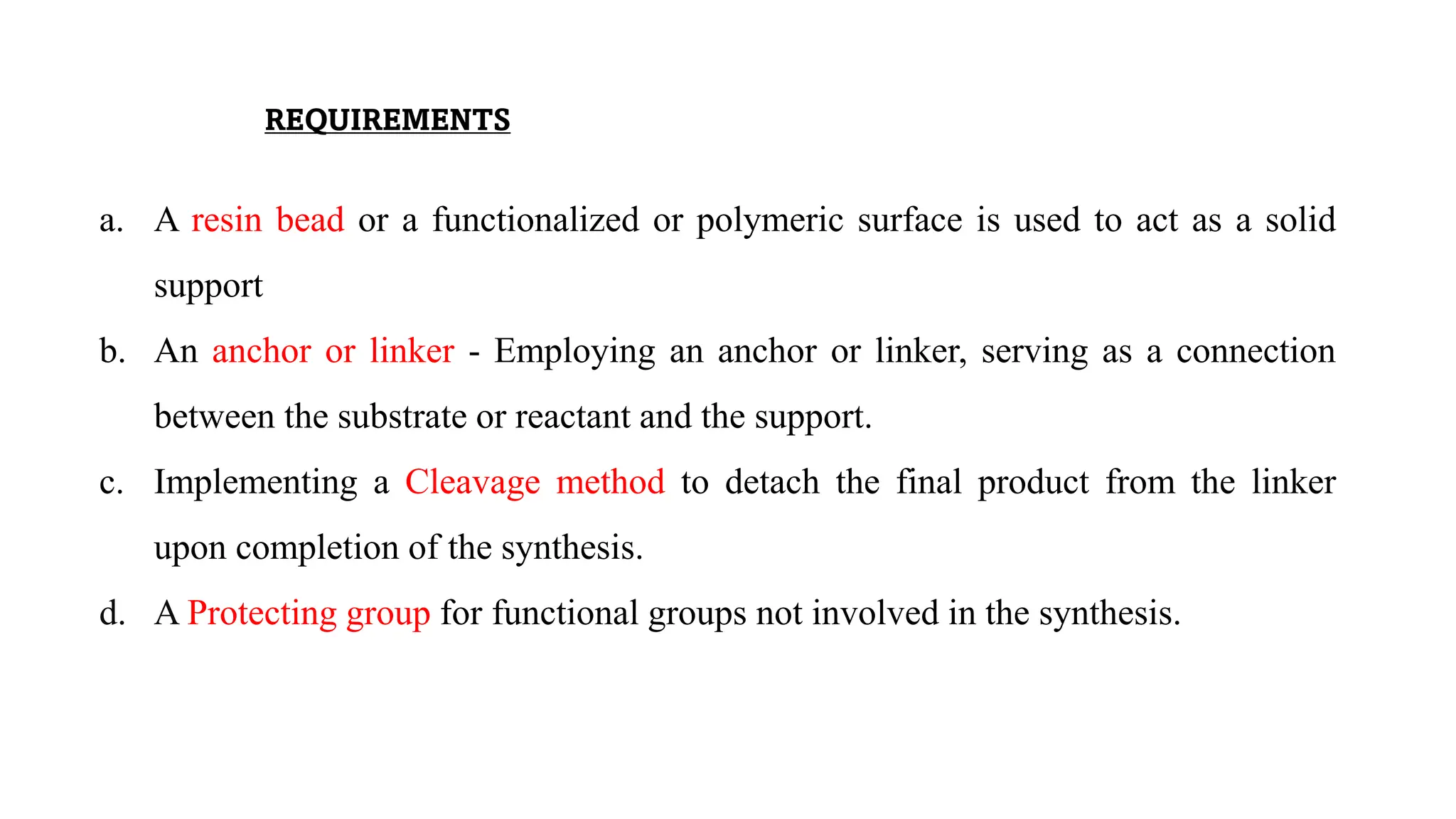
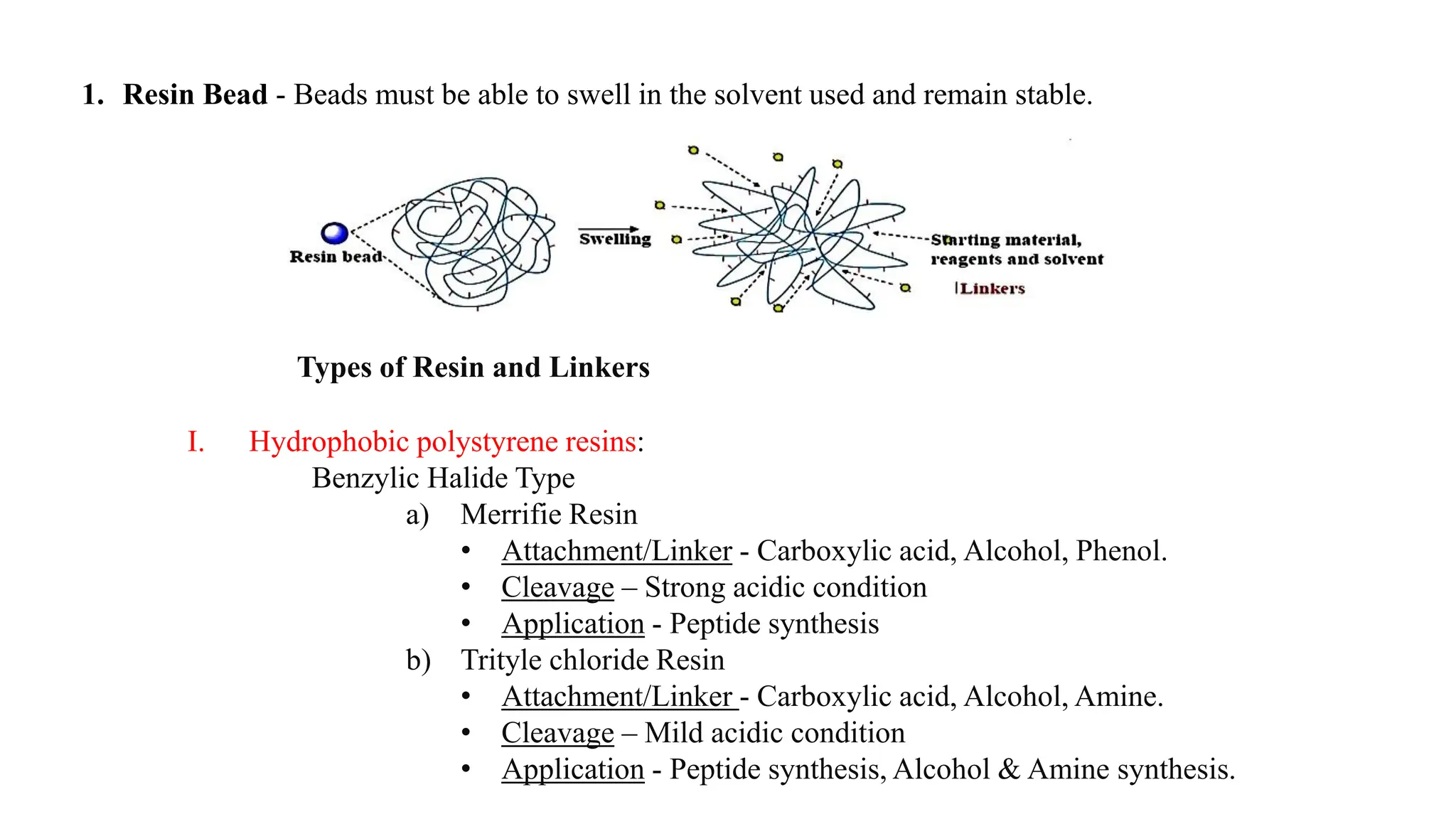
The document discusses lead identification in drug discovery through combinatorial chemistry and high throughput screening. It begins with an introduction to lead identification and defines it as identifying compounds with potential pharmacological activity against a target. It then describes two main methods for lead identification - combinatorial chemistry and high throughput screening. Combinatorial chemistry is defined as the rapid production of large numbers of similar molecules for screening, while high throughput screening allows rapid automated testing of large compound libraries to identify initial hits for further evaluation as potential drug leads.