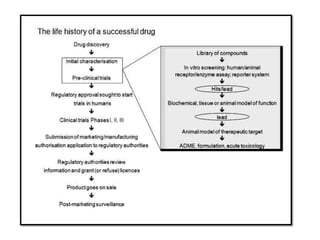
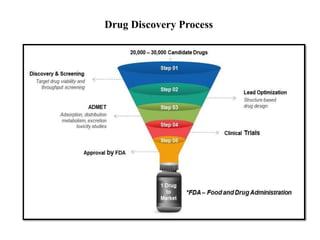
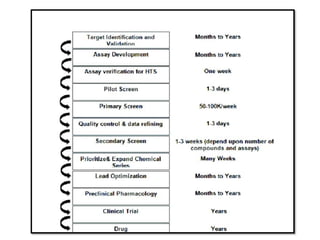
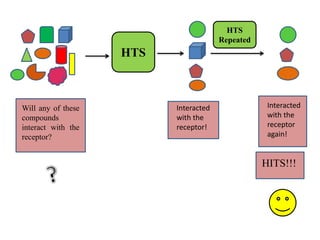
High Throughput Screening (HTS) is an automated method in drug discovery used to rapidly test large numbers of chemical compounds or biological entities to identify candidates with desired biological activity. It involves miniaturized assays and sophisticated technologies, enabling the screening of over 100,000 samples per day, significantly accelerating the process of identifying promising drug candidates. While HTS offers many advantages such as cost-effectiveness and efficiency, it also faces challenges including high costs and the complexity of data analysis.