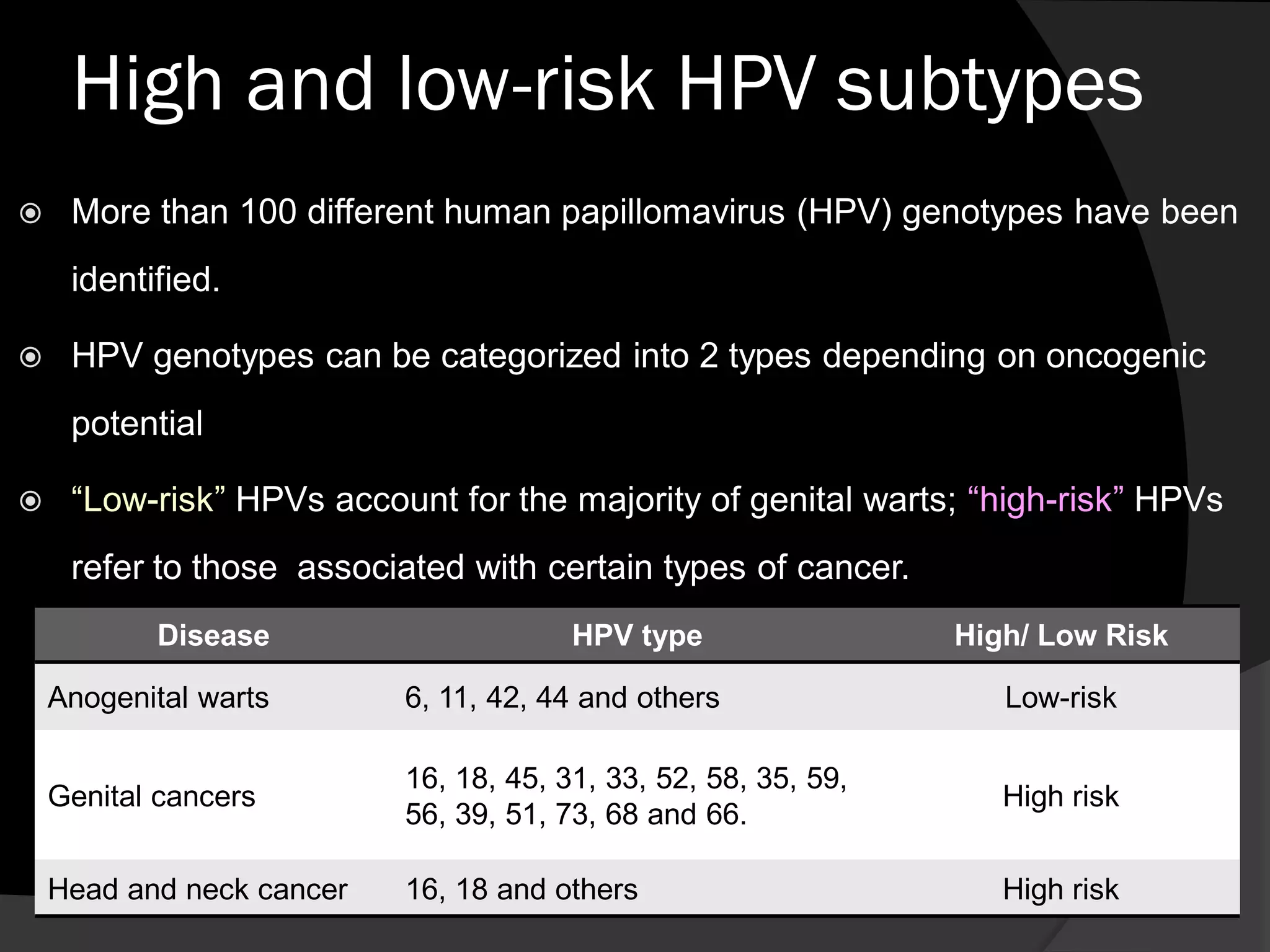
Cervical cancer is a major health problem, but HPV vaccination and screening can reduce rates. HPV is transmitted sexually and can cause cervical cancer. The vaccine protects against high-risk HPV types 16 and 18, which cause most cancers. Northern Ireland has high HPV vaccination rates. While the vaccine reduces cancer risk, screening is still needed since the vaccine does not protect against all HPV types and cannot help women already infected. In the future, screening may shift to HPV testing as prevalence decreases due to vaccination.