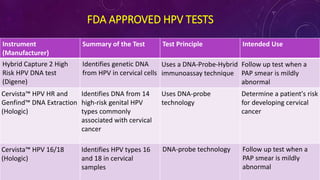
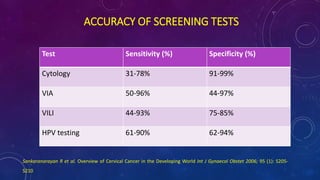
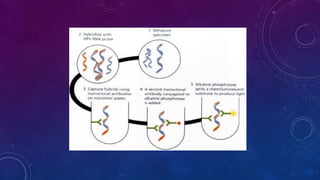
The document discusses HPV screening and co-testing, with a focus on the cobas® HPV test, which is FDA-approved for cervical cancer screening and is effective in detecting high-risk HPV types. It also outlines guidelines for cervical cancer screening, emphasizing the efficacy of primary HPV testing and co-testing in women, particularly those aged 25 years and older. The conclusion suggests that HPV primary screening is a promising advancement that could significantly improve cervical cancer detection and management.