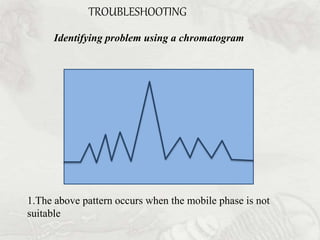
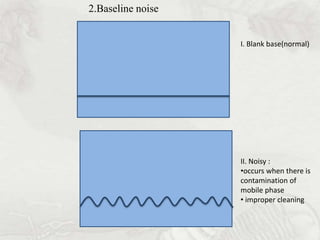
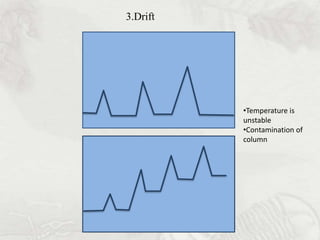
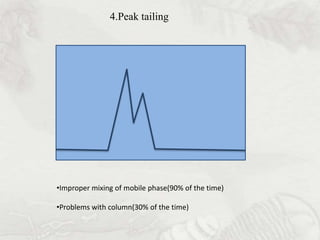
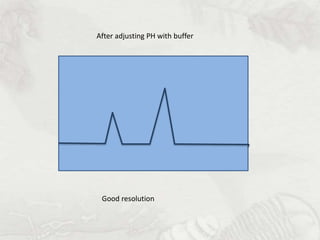
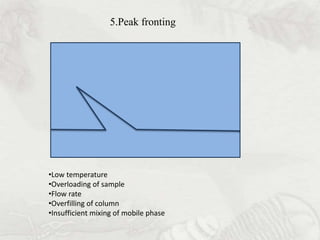
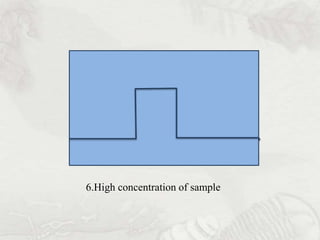
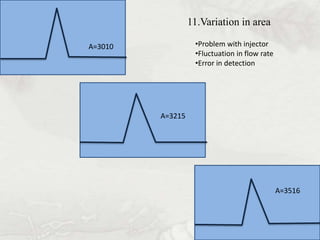
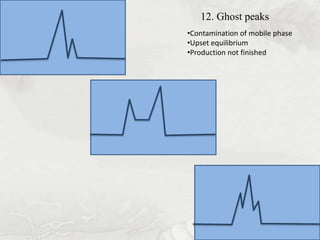
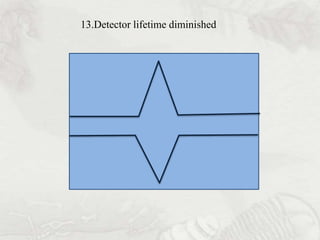
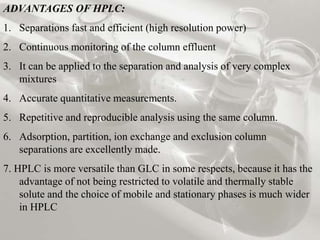
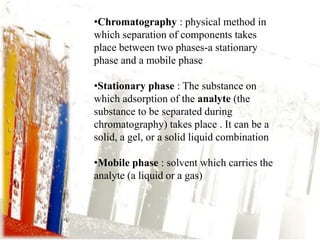
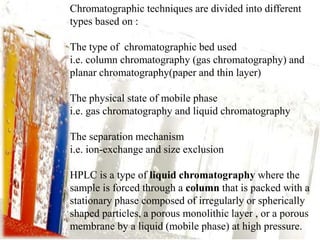
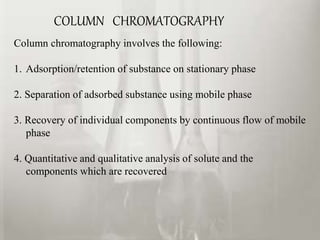
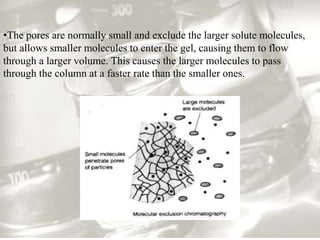
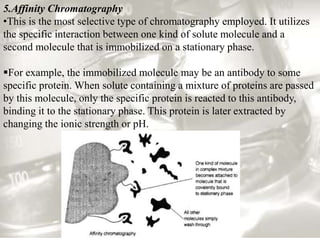
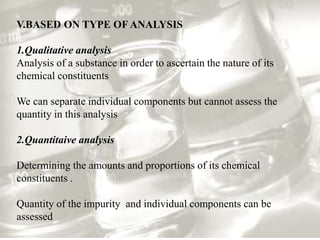
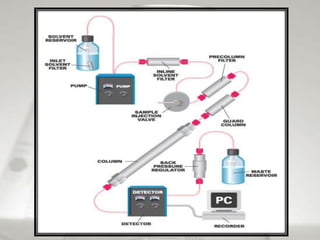
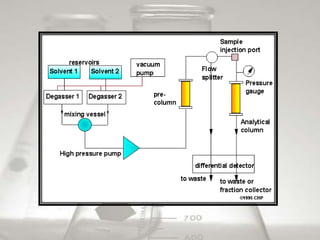
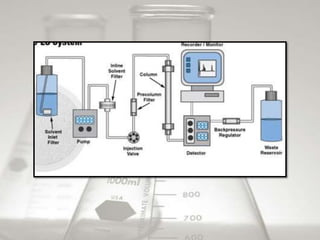
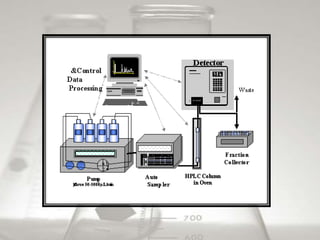
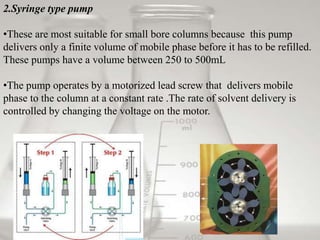
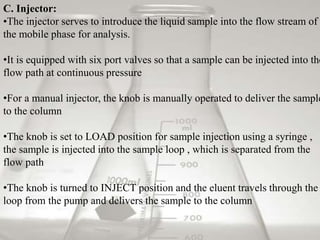
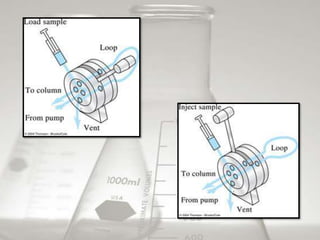
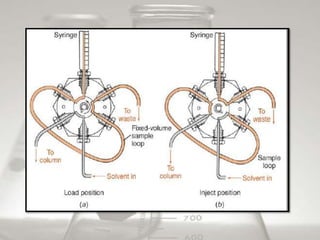
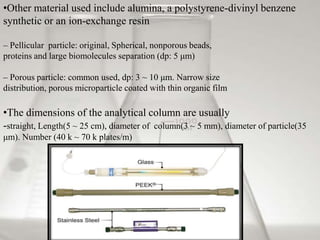
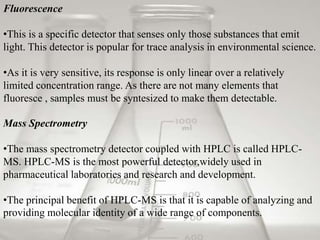
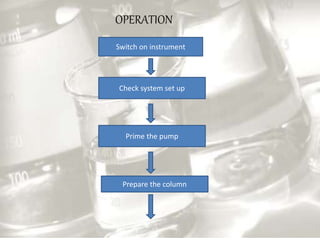
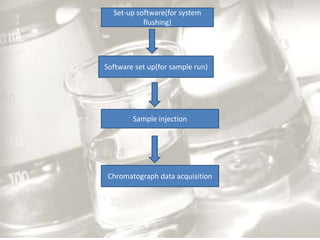
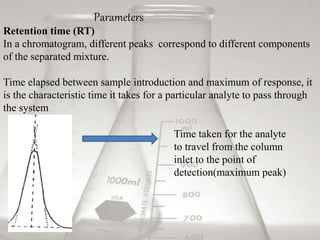
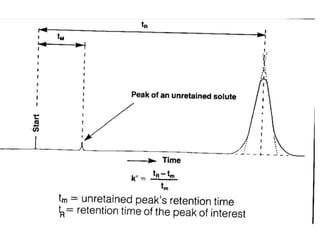
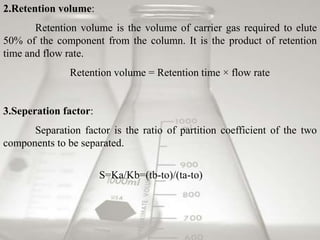
This document provides an overview of high-performance liquid chromatography (HPLC). It begins by defining HPLC and explaining that it uses small particle columns and high pressure to achieve faster separations compared to traditional liquid chromatography. The document then discusses the basic components and principles of HPLC, including the stationary and mobile phases, various modes of separation, and common instrumentation such as pumps, injectors, columns, and detectors. It provides details on the configuration and function of each component.





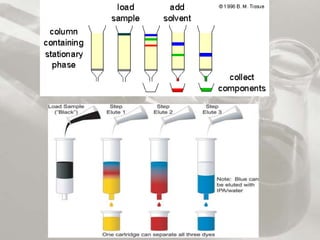



































![Preparation of stock solution:
•Take 50mg of caffeine and transfer into 50ml of flask and make it up
to the mark with the diluent
•Caffeine is used a it gives a multiwavelength response and is stable
•Prepare solutions of different PPM (100-600)
•Inject them and calculate the area
•Correlation coefficient r is used to check the detector linearity and
cannot be less than .999
• r = NƩXY-ƩXƩY/√ [NƩX²-(Ʃx) ²][NƩY²-(ƩY)²]
•The graph obtained between concentration and area is linear](https://image.slidesharecdn.com/hplc-140913081220-phpapp02/85/Hplc-c-72-320.jpg)