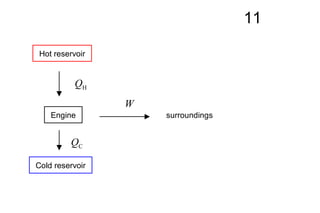
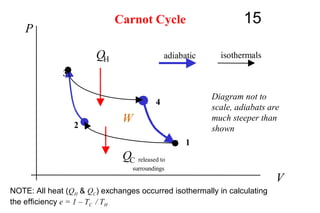
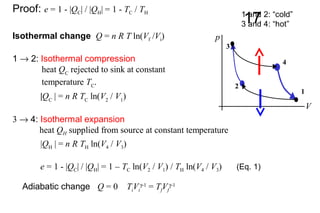
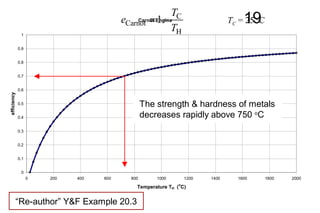
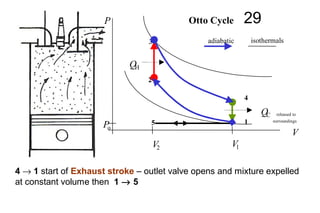
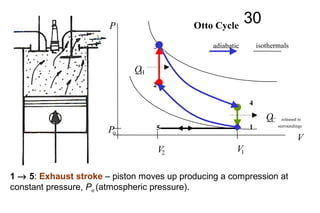
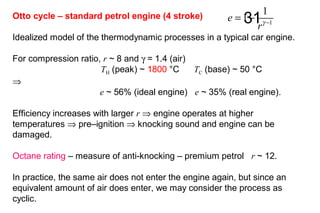
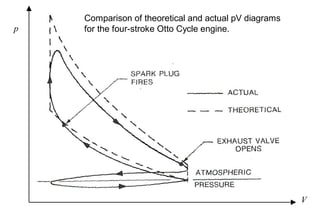
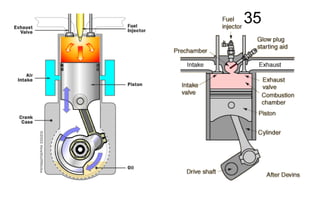
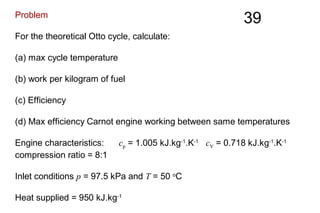
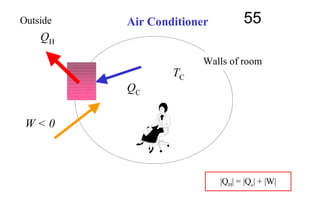
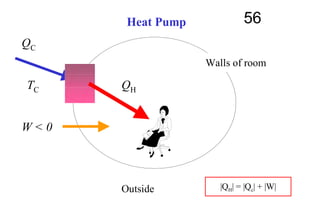
The document discusses thermal physics, specifically focusing on heat engines, including the Carnot cycle and Otto and Diesel engines. It explains the principles of heat transfer, work output, and thermal efficiency, highlighting calculations for specific problems involving engine performance. Additionally, it addresses the limitations of heat engines in achieving 100% efficiency due to the second law of thermodynamics.