Embed presentation
Download to read offline






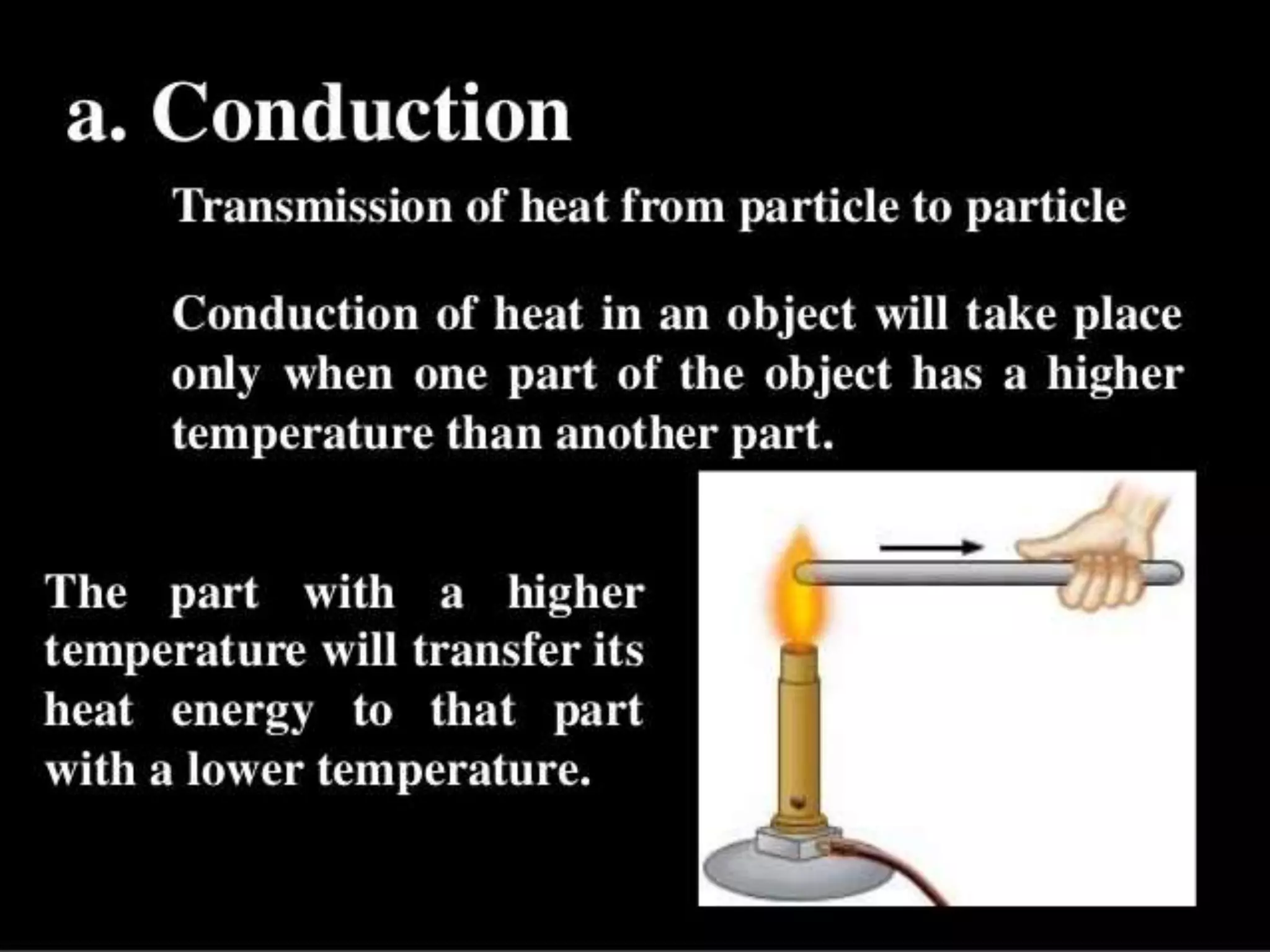
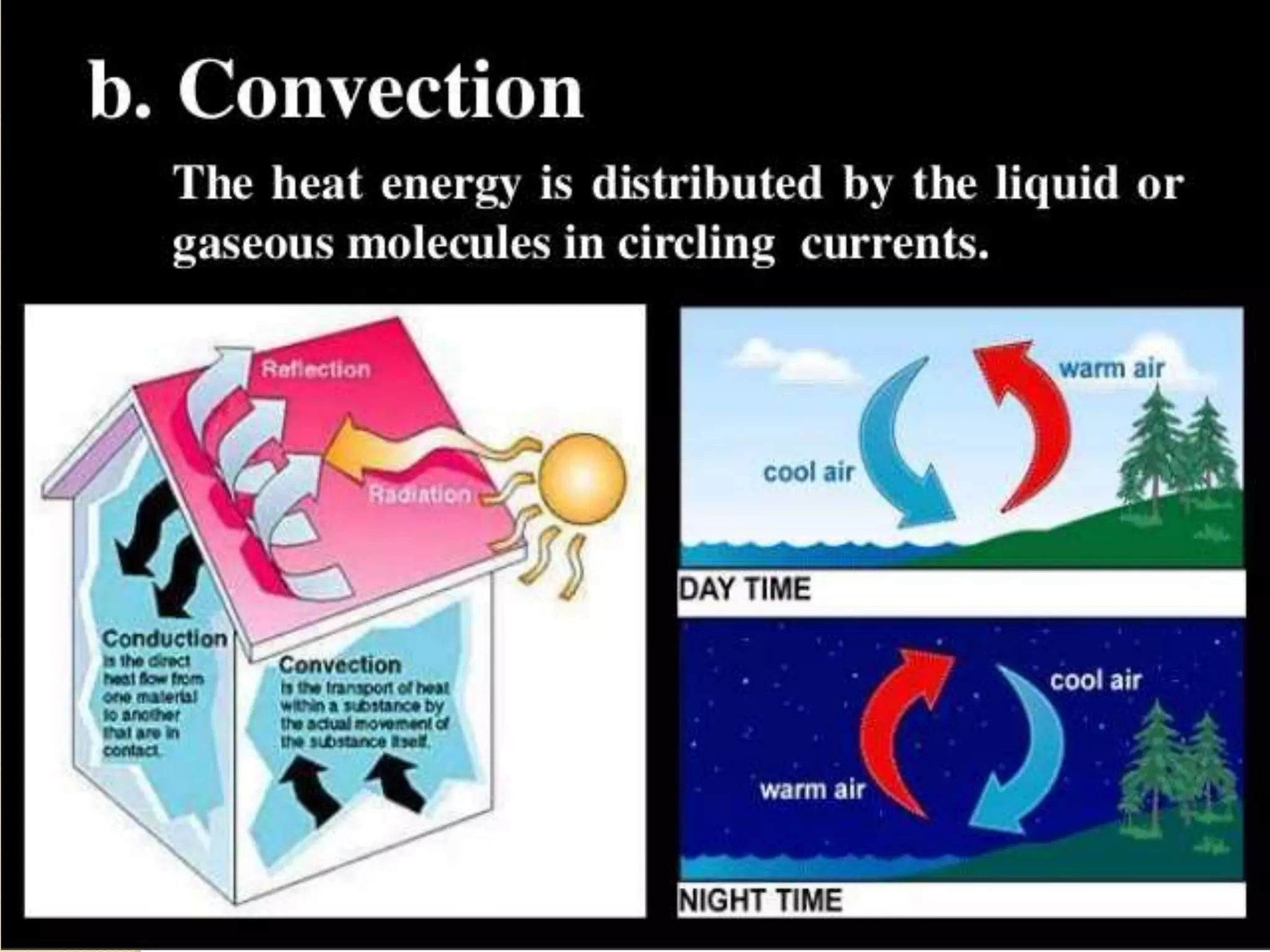
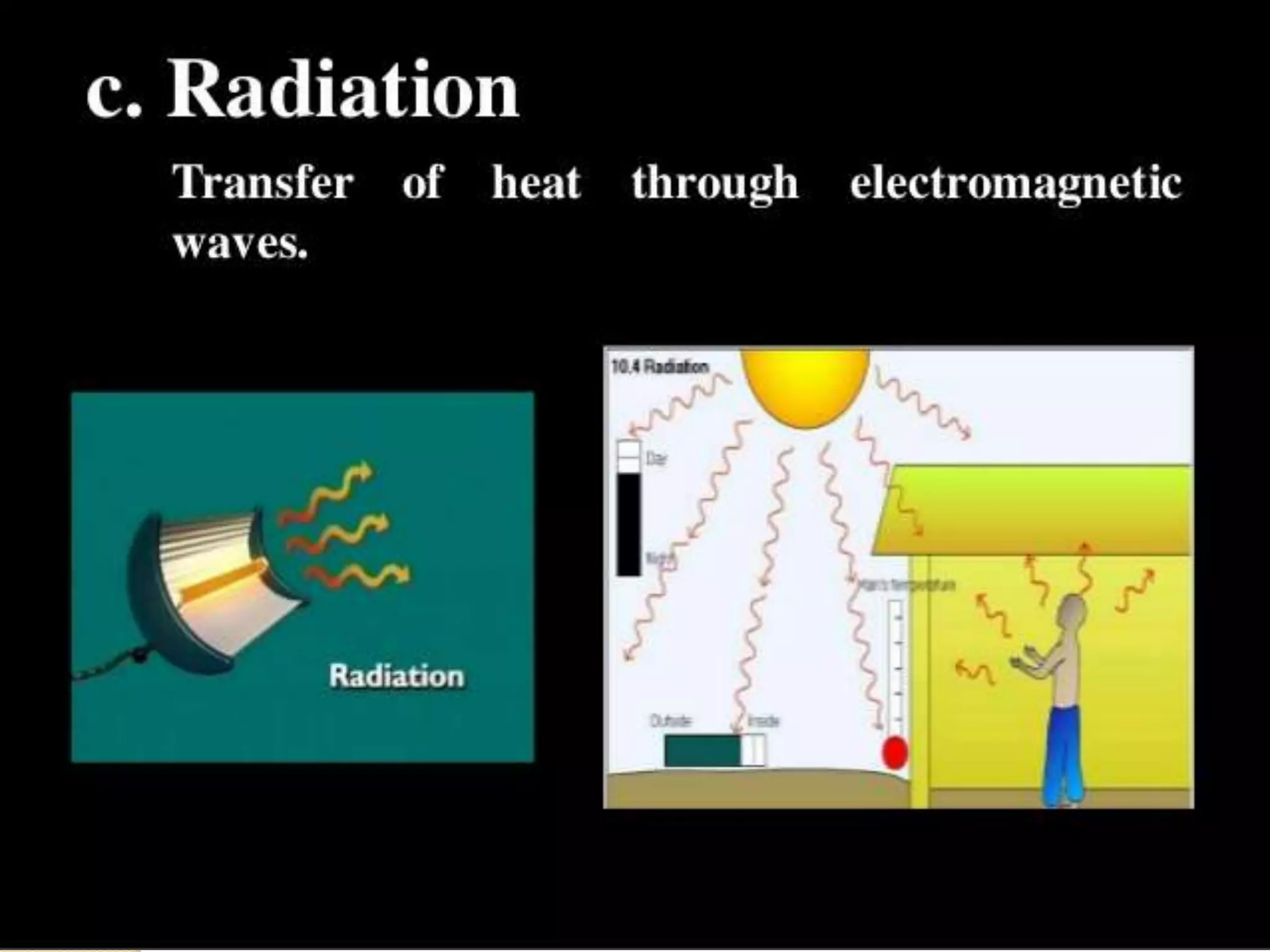

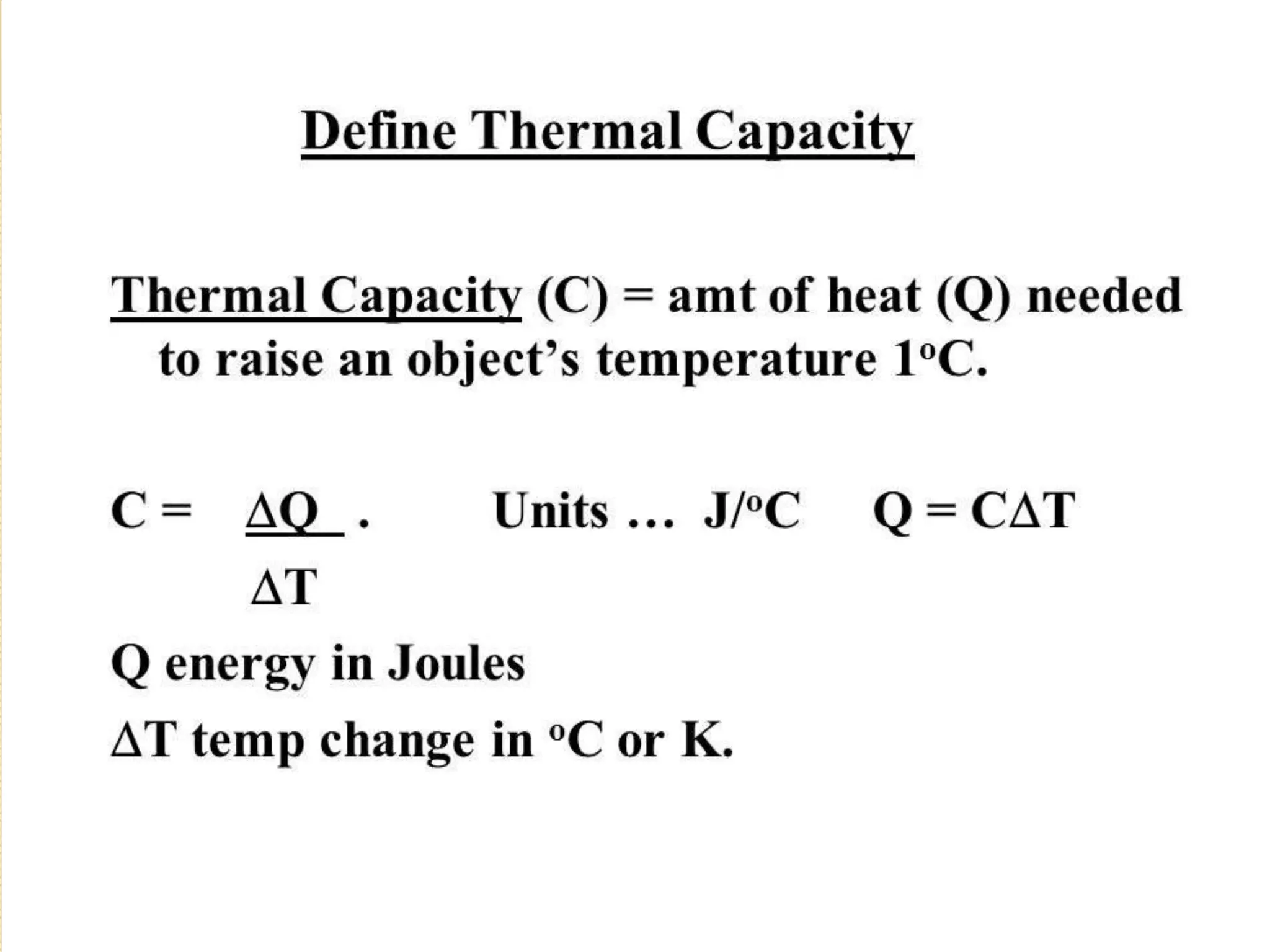
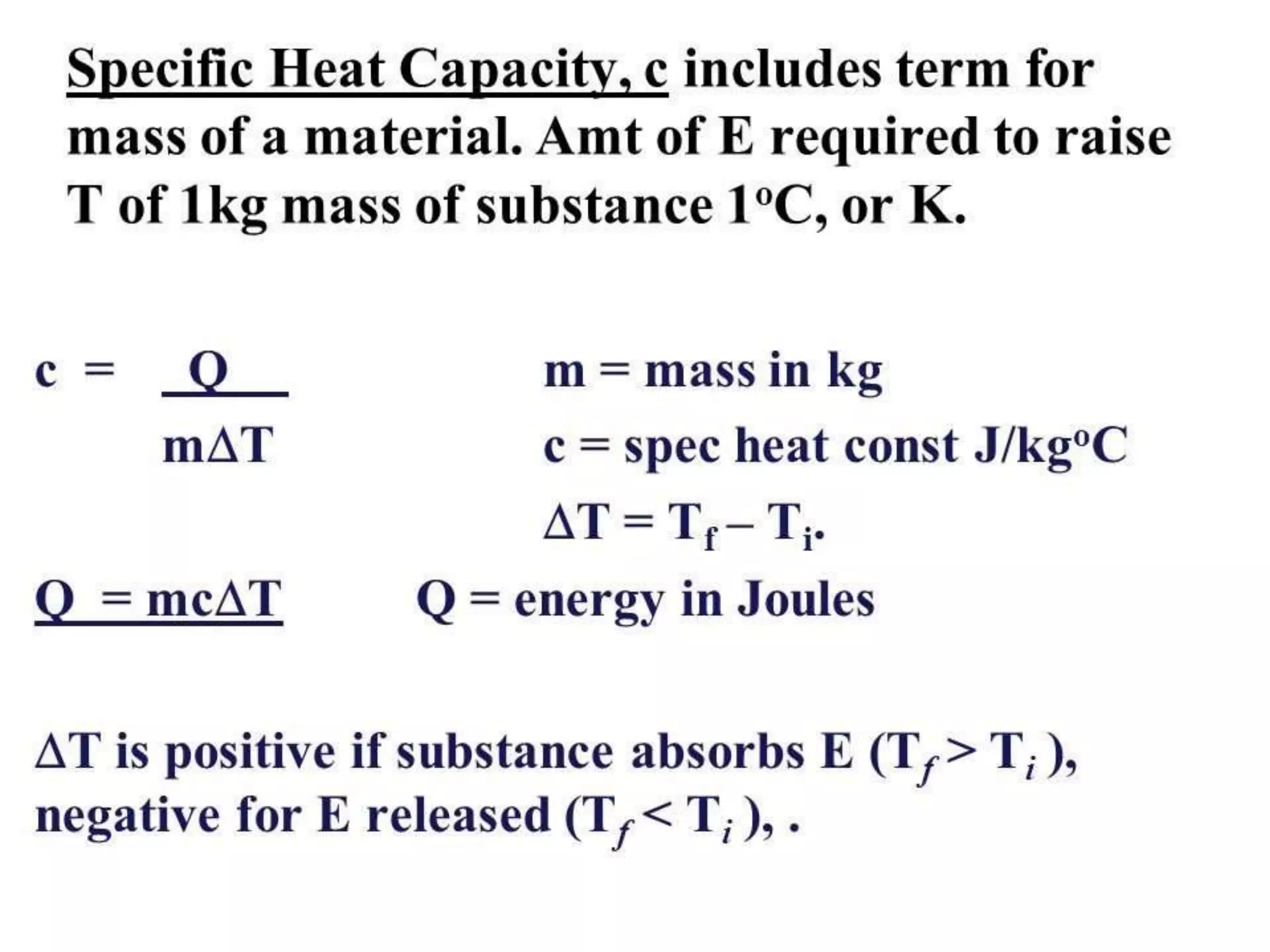

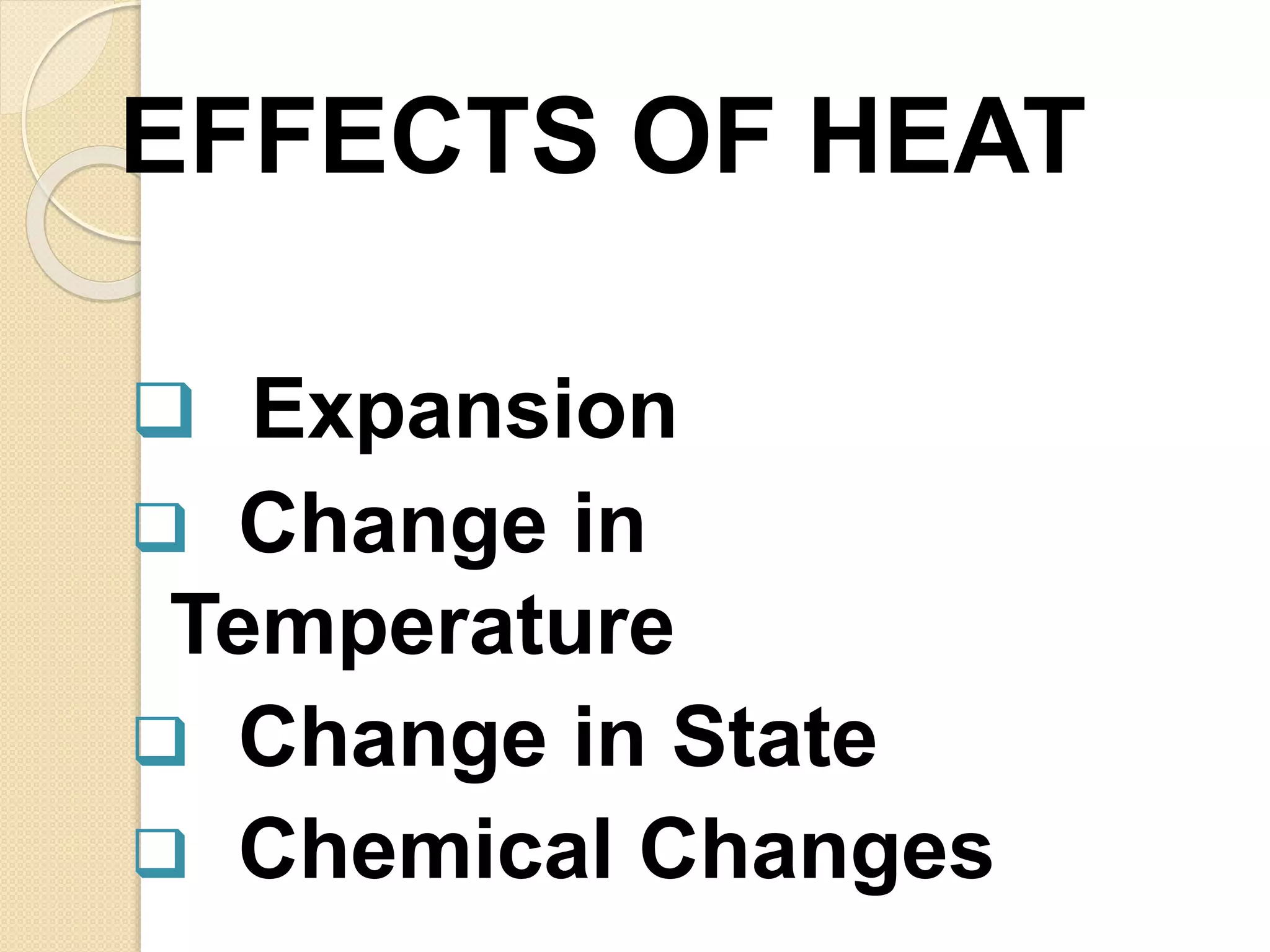


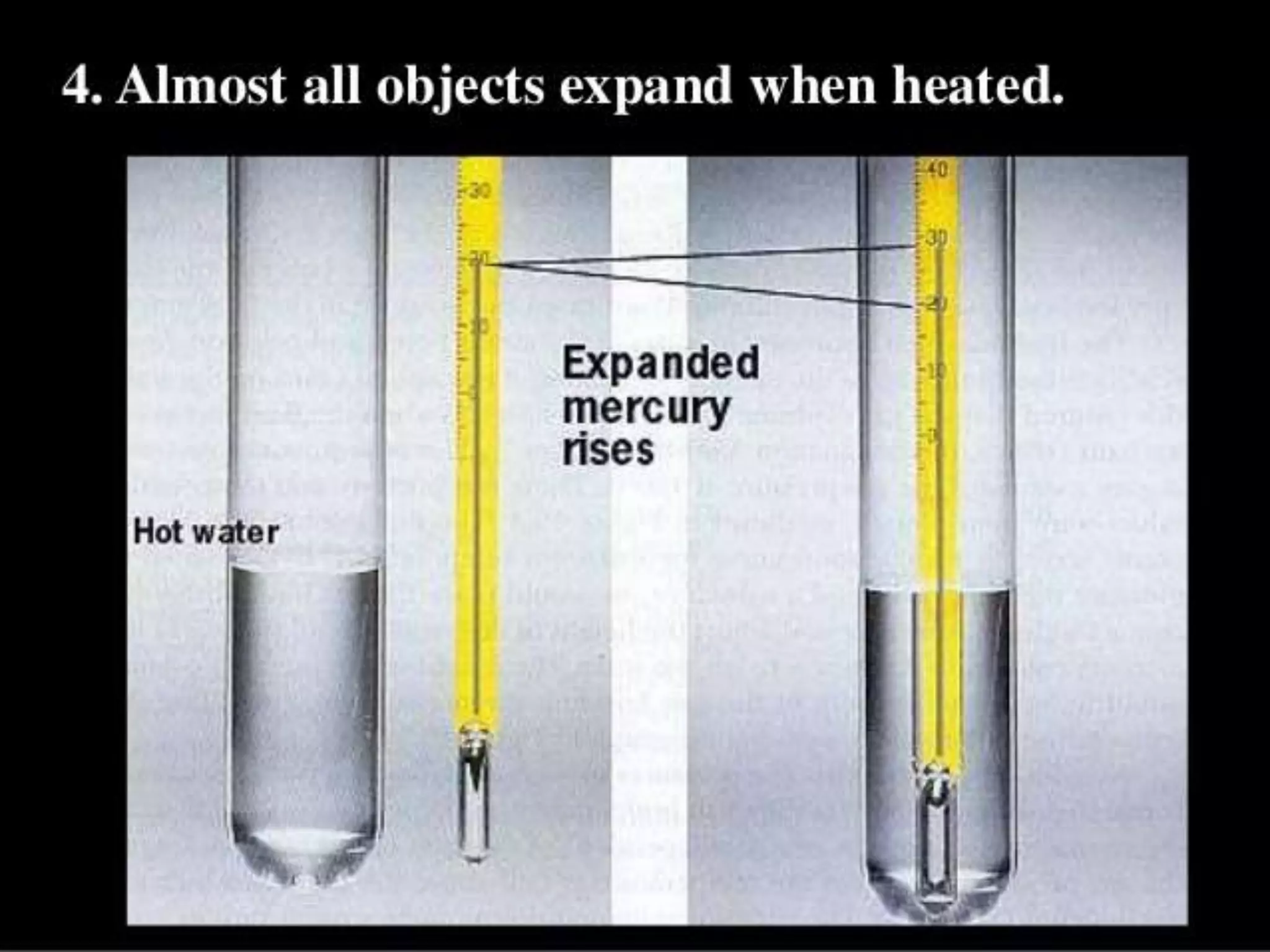


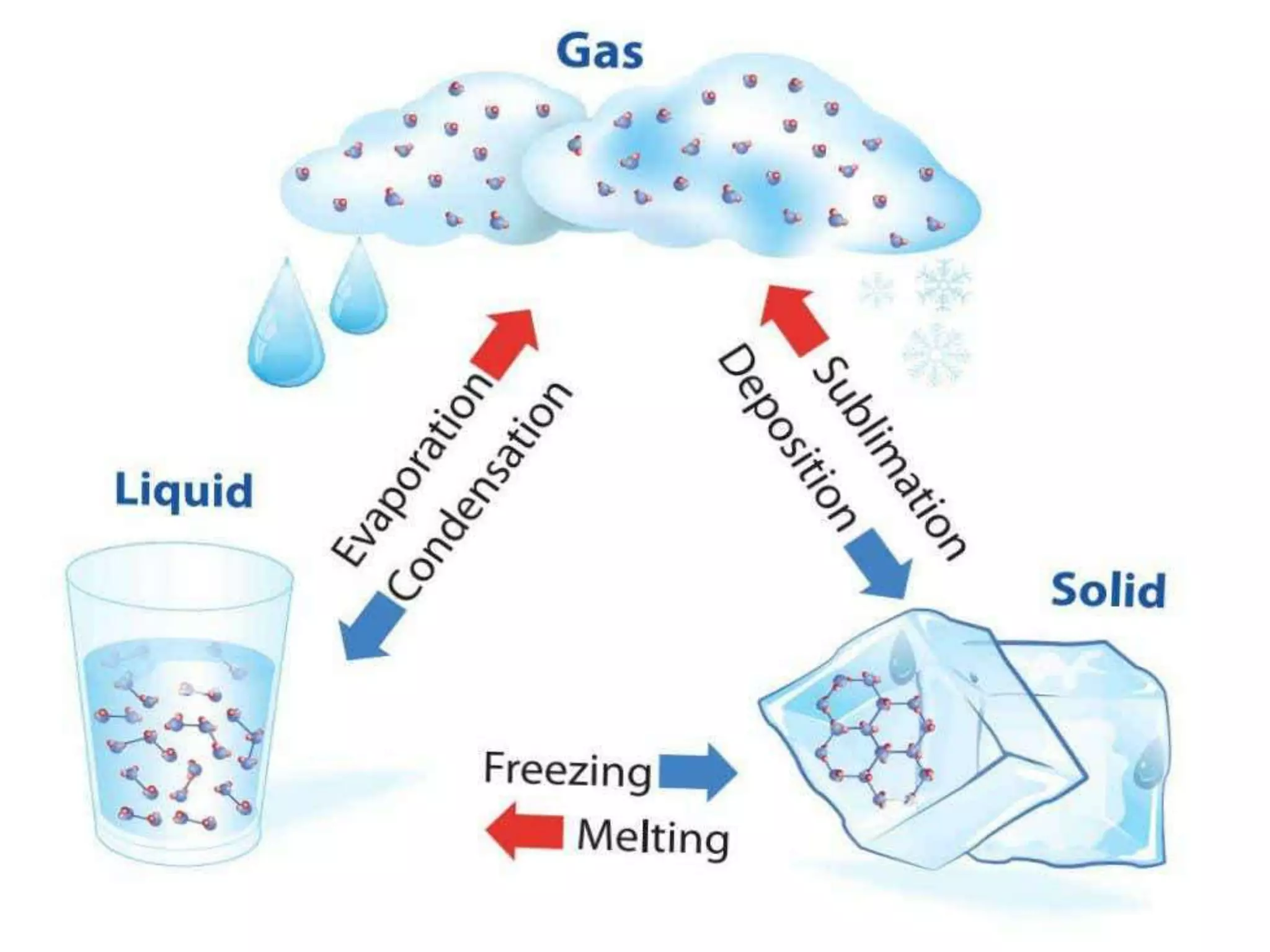
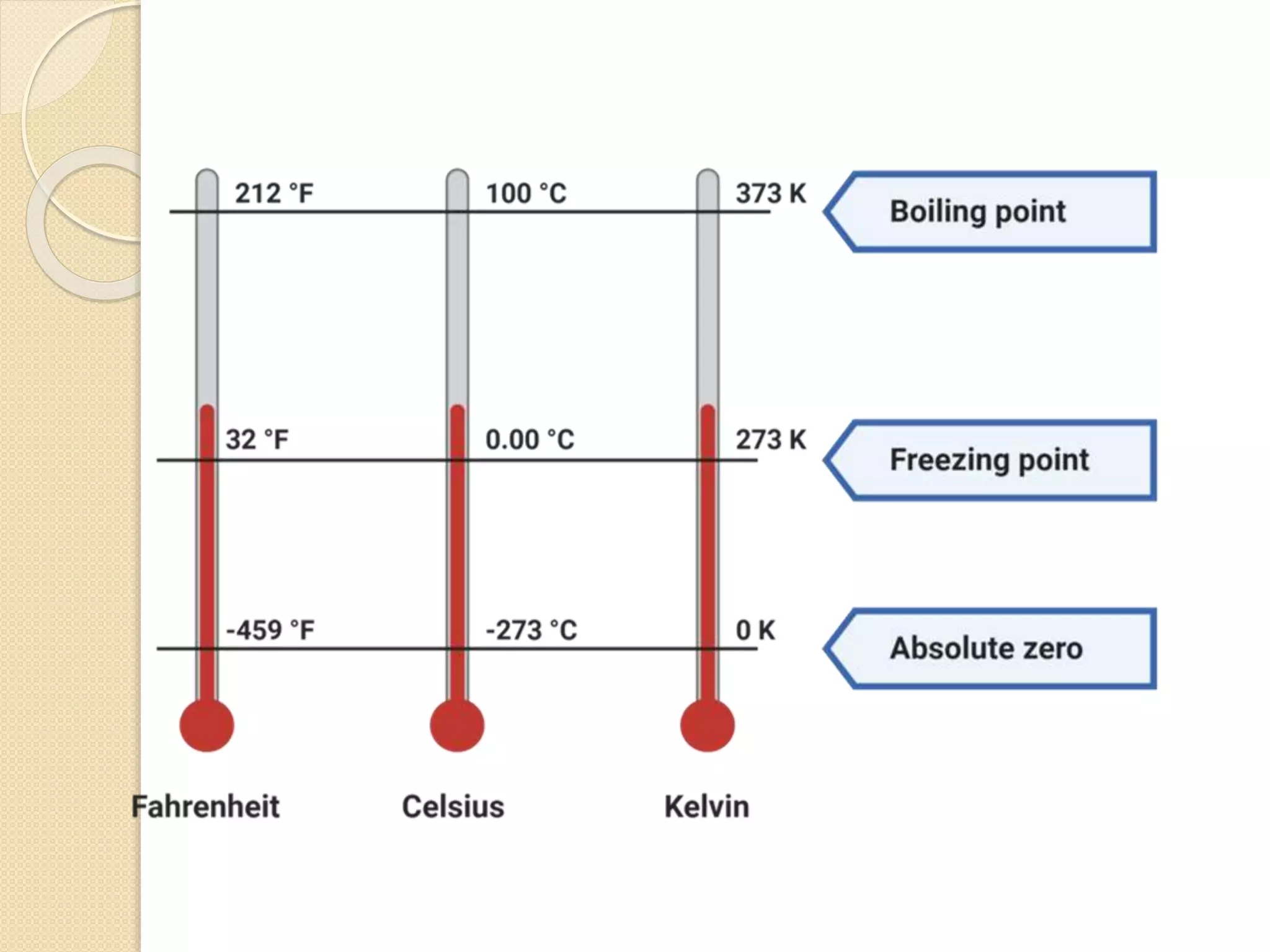
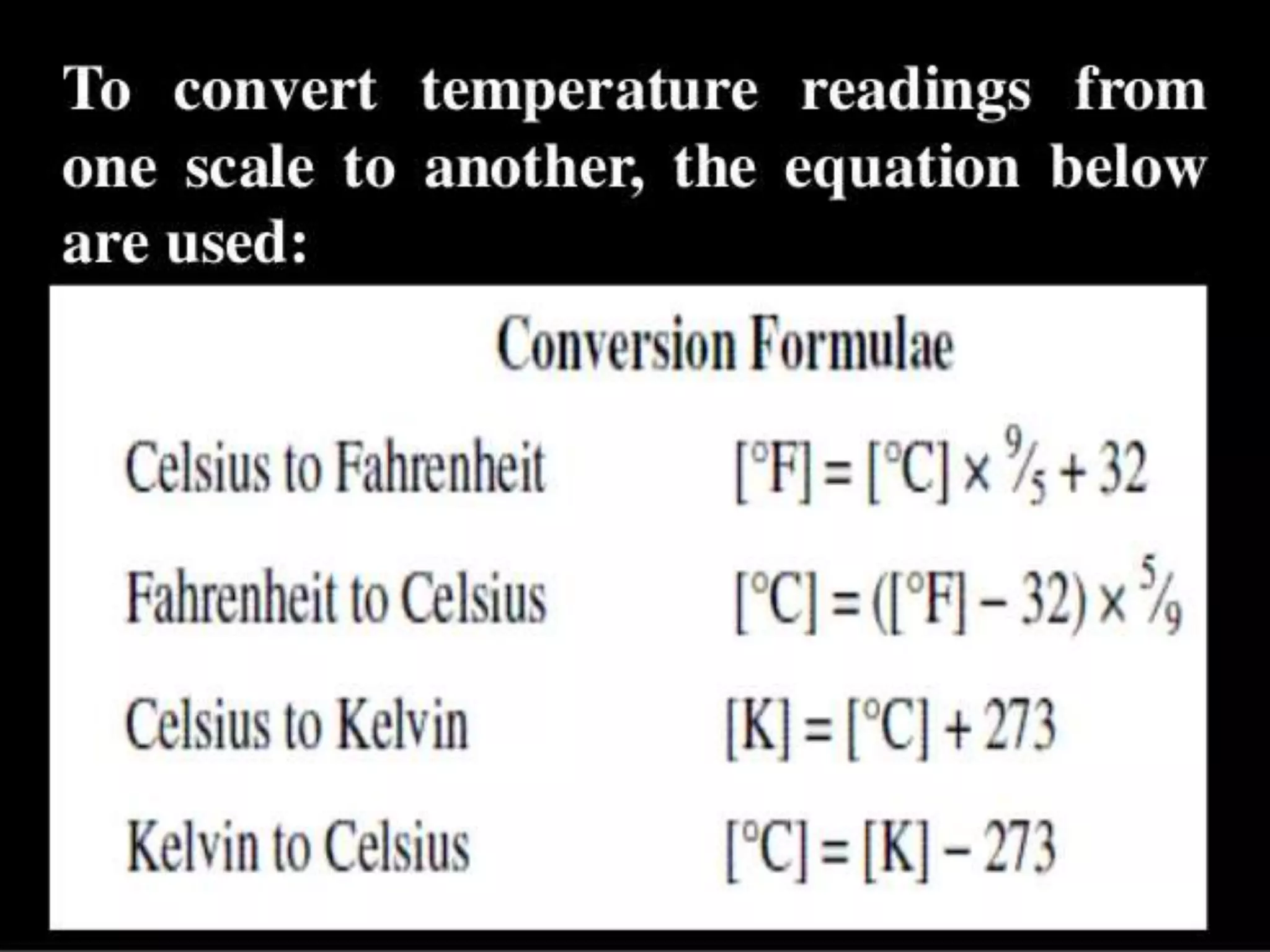
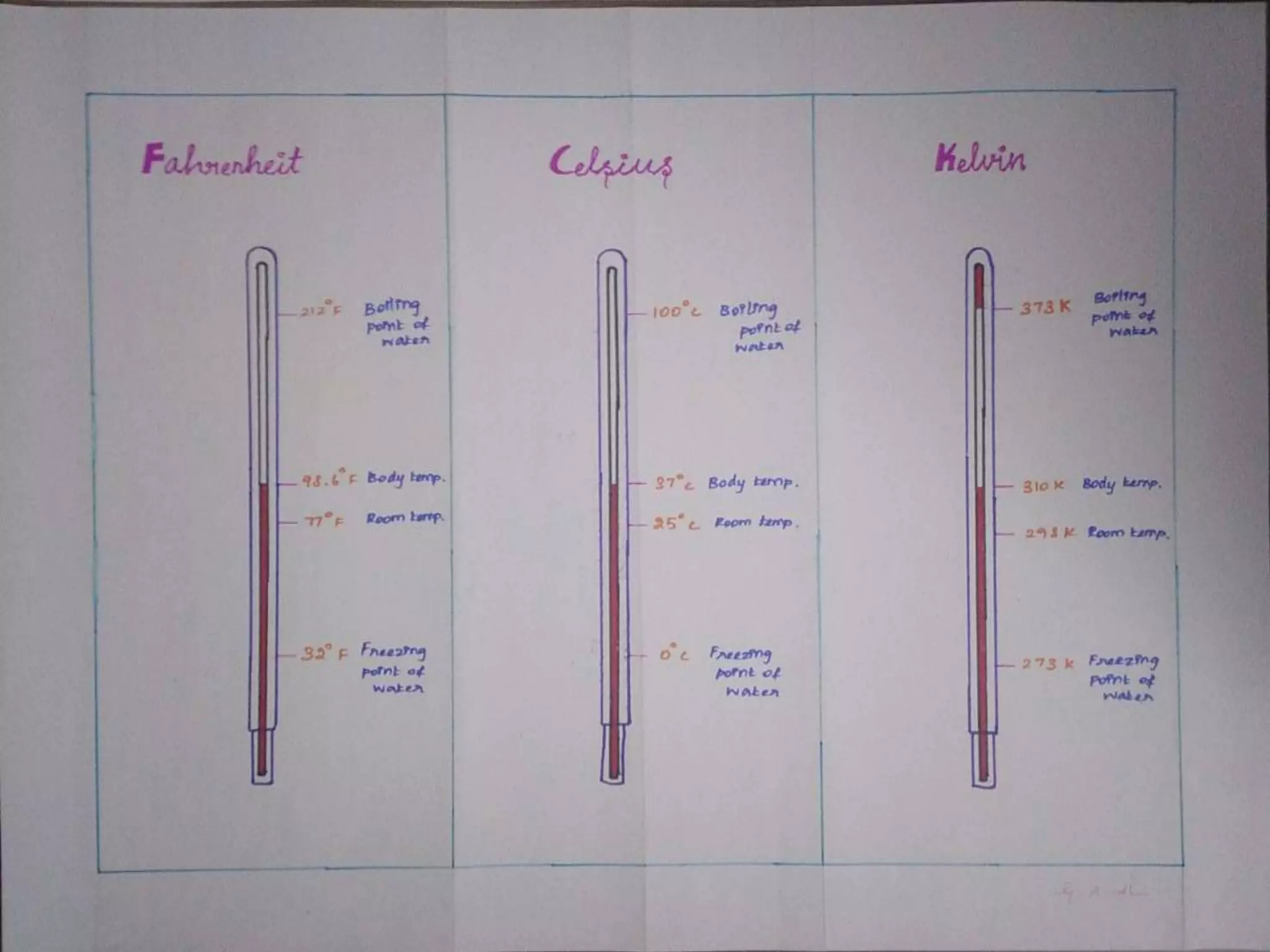
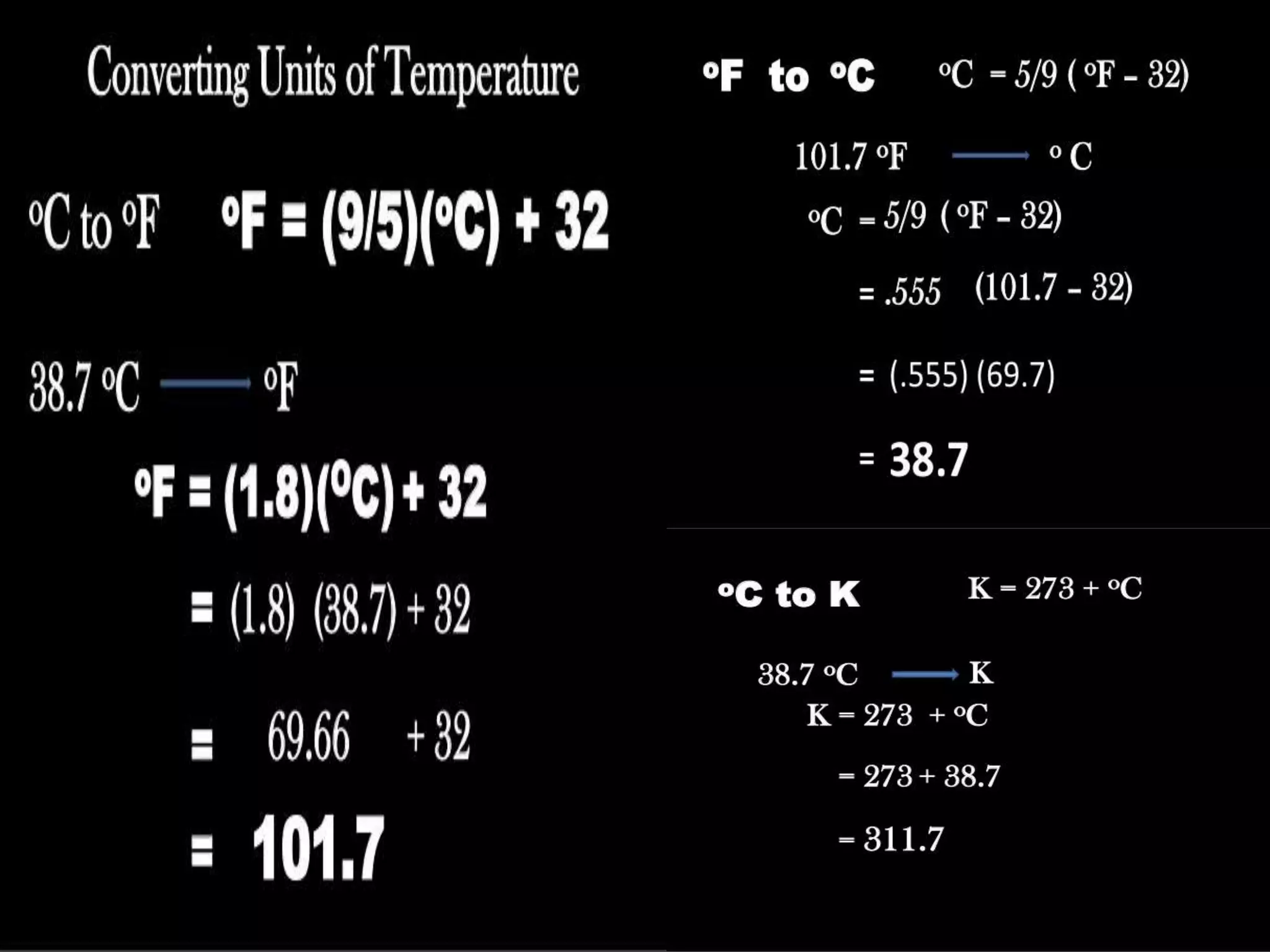



The document discusses key concepts relating to heat and temperature. It defines heat as the spontaneous flow of energy from objects at a higher temperature to those at a lower temperature. Temperature is defined as the degree of hotness or coldness of a body. Different temperature scales such as Fahrenheit, Celsius, and Kelvin are also discussed. The document also covers heat capacity, specific heat capacity, and the various effects of heat such as expansion, changes in temperature and state, and chemical changes.




























