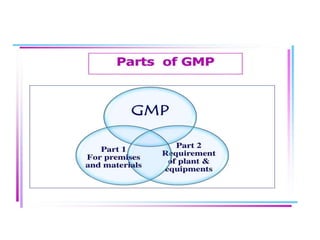
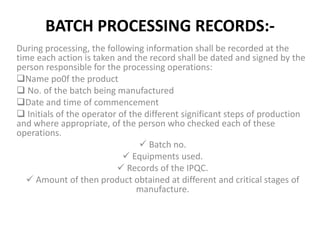
The document discusses Good Manufacturing Practices (GMP) for pharmaceutical manufacturing. It outlines requirements for facilities, equipment, personnel, documentation, production processes, quality control, and other operational aspects to minimize risks and ensure consistent production of quality products. GMP covers all aspects of production and testing to maintain standards, prevent contamination and errors, and comply with regulatory guidelines.