This document discusses good manufacturing practices for manufacturing operations and controls in the pharmaceutical industry. It covers several key topics:
1. Sanitation of manufacturing premises is important to ensure good hygiene of facilities, equipment, processes, and personnel. Cleaning and validation procedures should be established and records maintained.
2. Proper controls must be established to prevent mix-ups and cross-contamination during production. This includes separation of products, labeling, cleaning procedures, and qualified personnel.
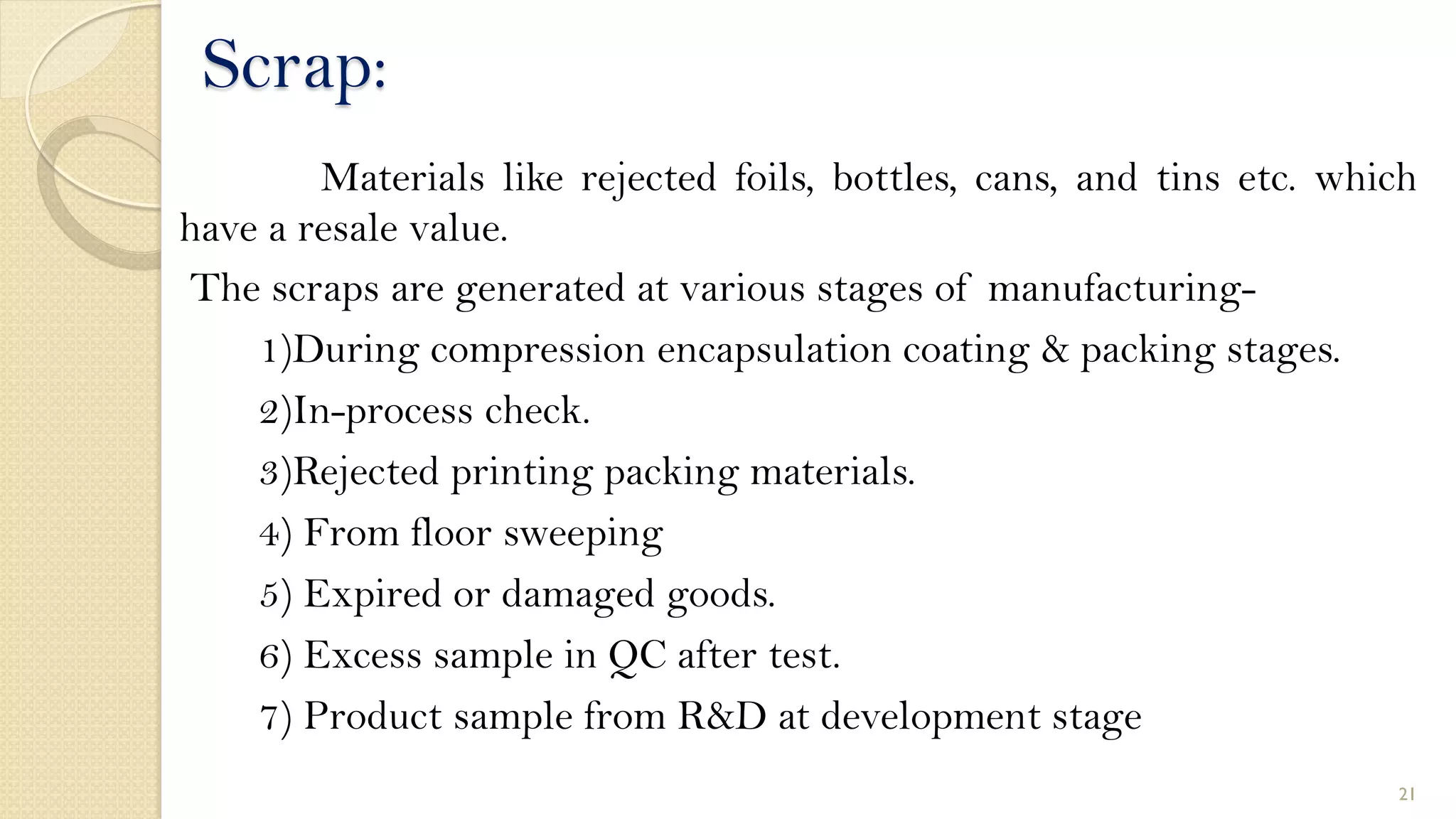
3. Waste and scrap from manufacturing must be properly handled, collected, stored, and disposed of according to established guidelines. Hazardous and pharmaceutical wastes require special treatment and disposal.