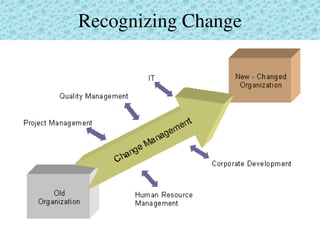
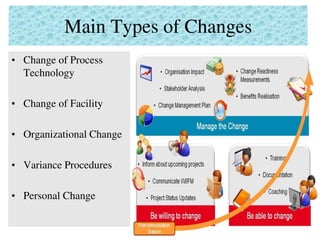
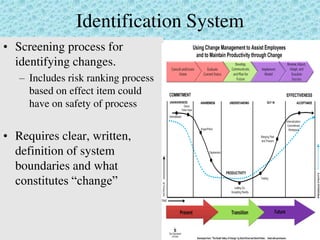
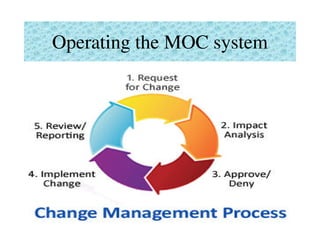
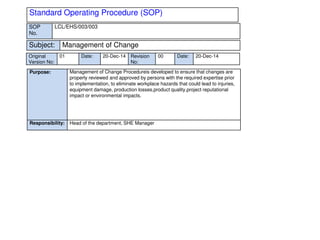
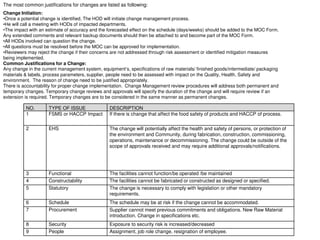
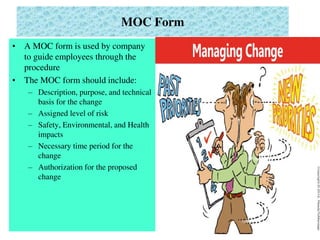
This document provides an overview of management of change (MOC) programs. It defines MOC as policies and procedures to ensure changes do not result in unsafe operations. The document discusses why MOC is needed, as unmanaged changes have historically led to major accidents. It outlines key elements of an effective MOC program, including identifying changes, approval mechanisms, training, and auditing. The document emphasizes that change is inevitable but must be carefully managed to maintain safety. An effective MOC program requires management support, understanding of procedures, and keeping stakeholders informed during the change process.