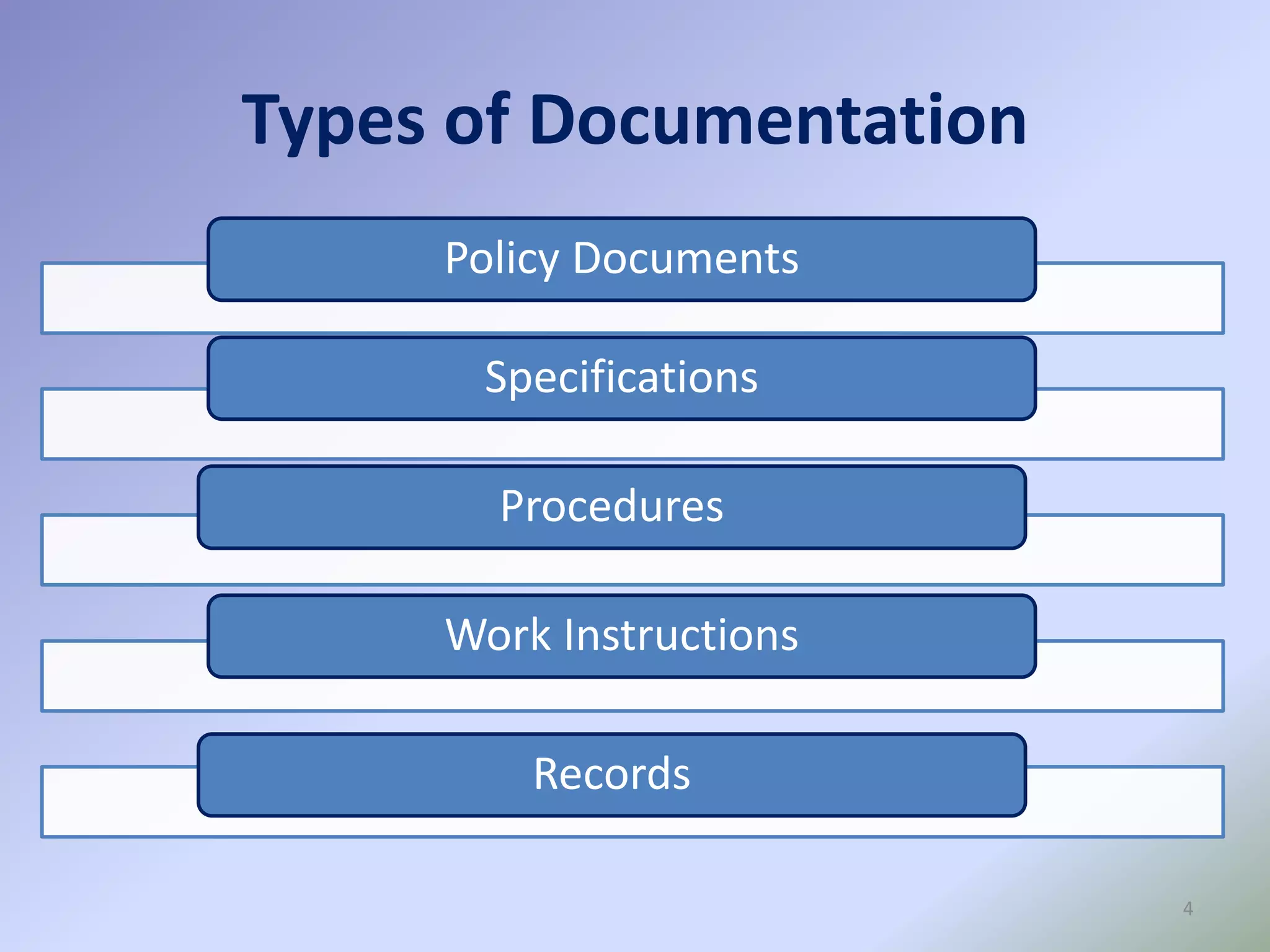
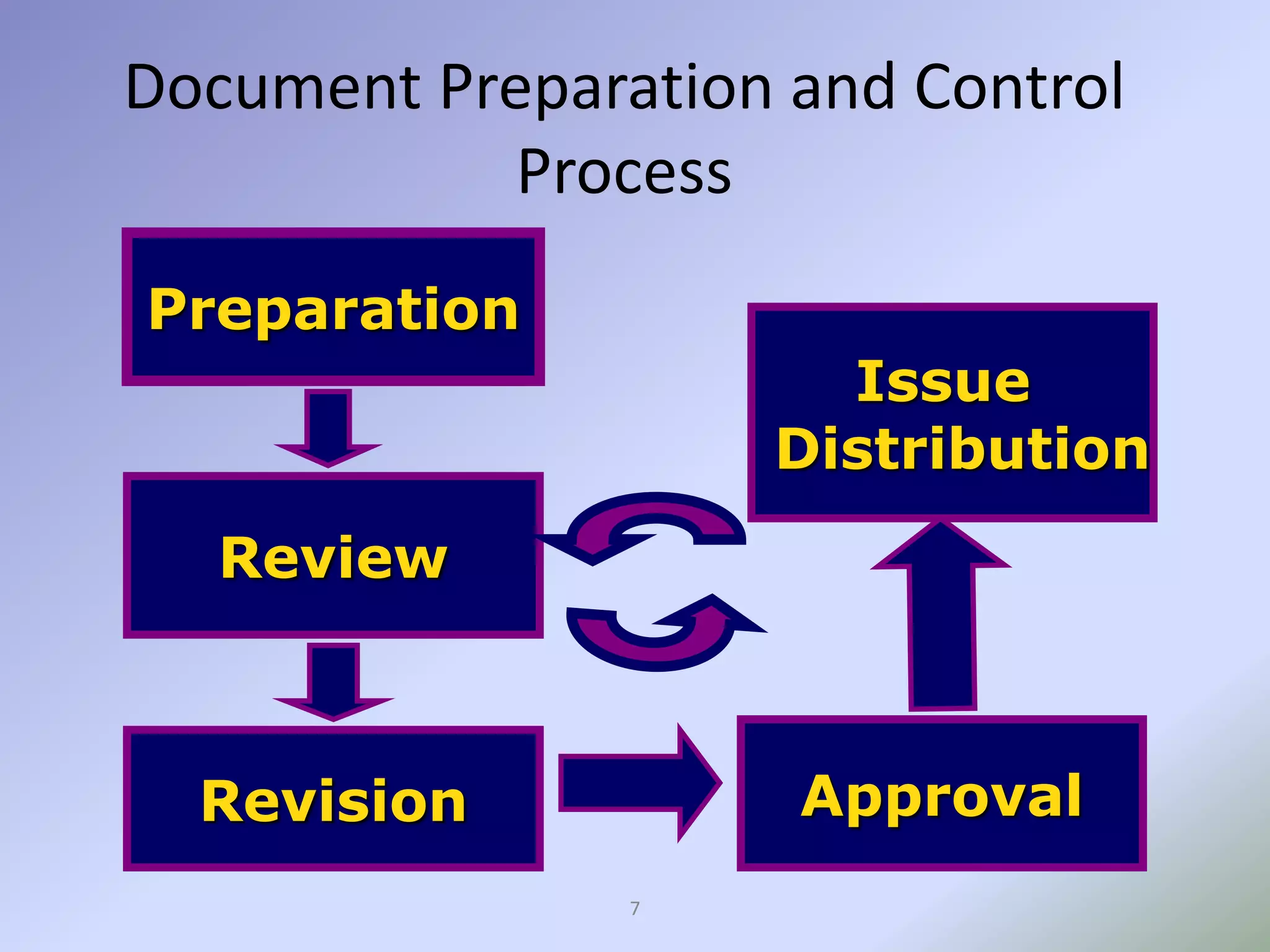
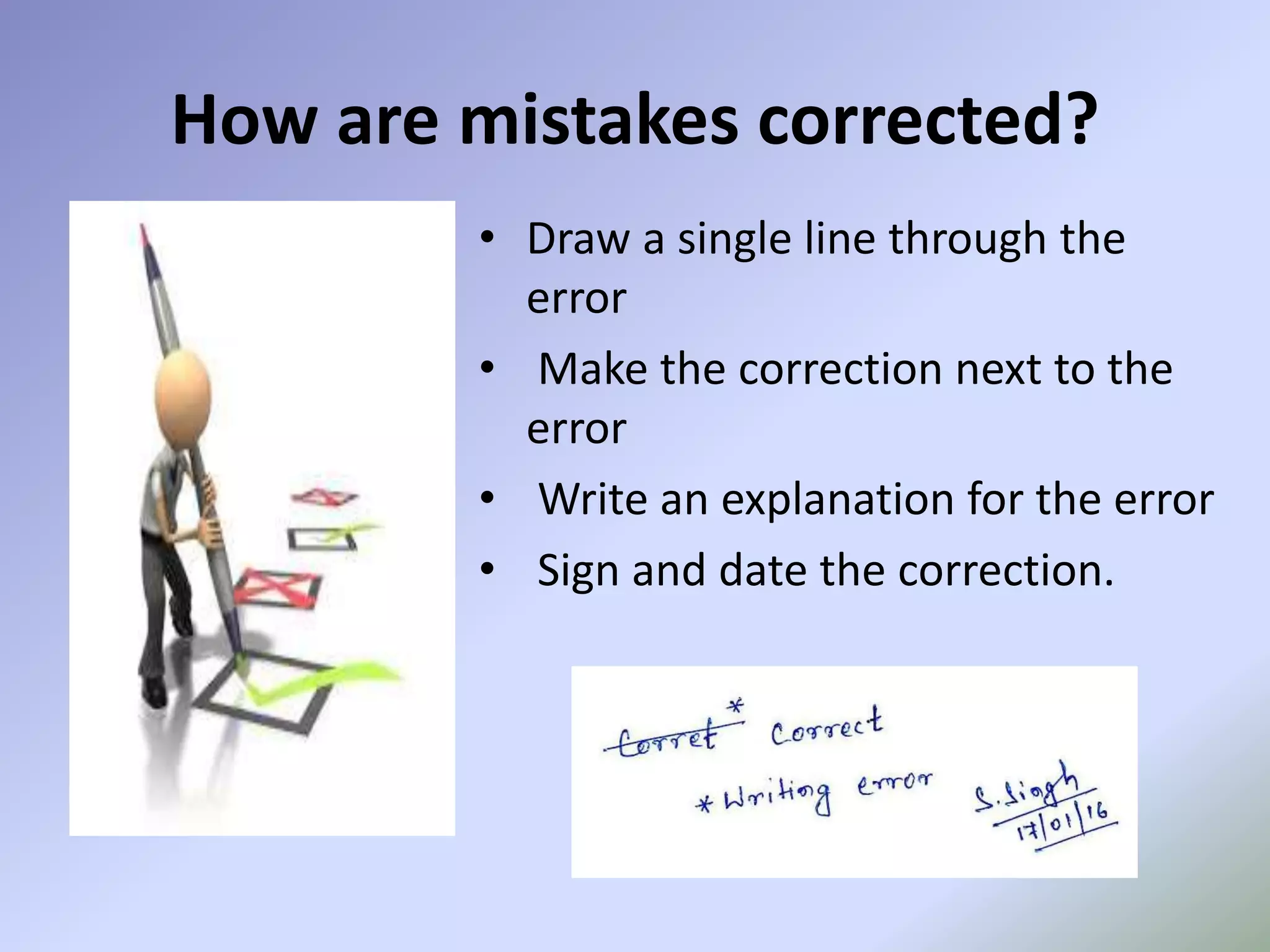
This document discusses pharmaceutical documentation. It defines documentation and its aims which include defining specifications, procedures, and ensuring authorized persons have necessary information. There are different types of documentation like policy documents, specifications, procedures, work instructions, and records. The document also outlines the documentation process including preparation, review, approval, distribution and revision. It describes how mistakes are corrected in documentation and concludes that documentation provides a basis for planning future manufacturing functions in a GMP environment.