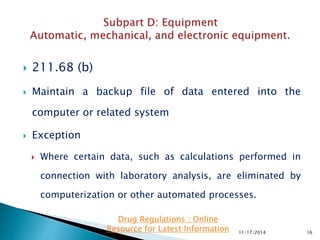
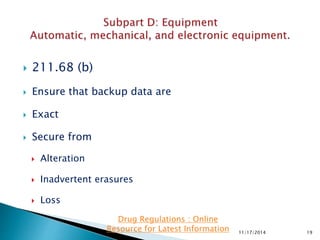
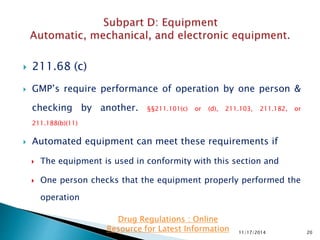
The presentation by 'Drug Regulations', a nonprofit organization, provides insights into the current good manufacturing practices (GMP) as per the US FDA guidelines for pharmaceuticals. It covers essential aspects such as equipment design, maintenance, and cleaning procedures needed to ensure the safety and quality of drug products. For more information and resources, individuals are directed to visit http://www.drugregulations.org.