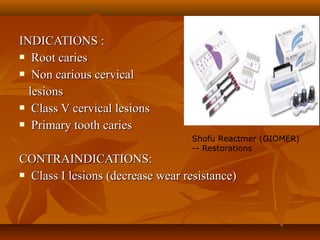
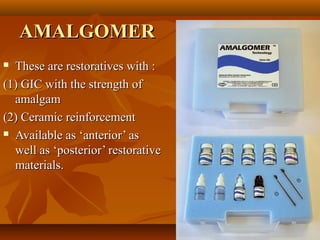
This document provides an overview of glass ionomer cement (GIC), including its composition, classification, setting mechanism, applications and uses, advantages/disadvantages, and modifications/advancements. Specifically:
- GIC is composed of fluoroalumino silicate glass powder and an ionic polymer of polyacrylic acid. It sets via an acid-base reaction between the glass and polymer.
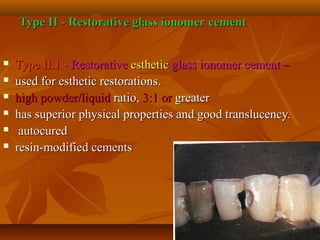
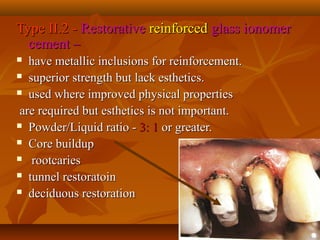
- GIC is classified based on its powder/liquid ratio and intended use, such as luting cement (Type I), restorative cement (Type II), or lining/base cement (Type III).
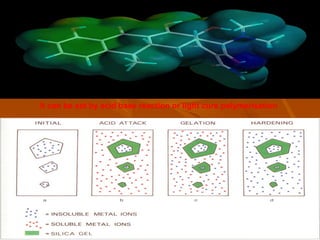
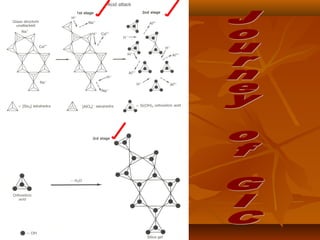
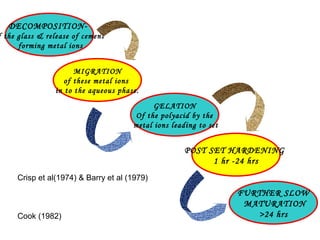
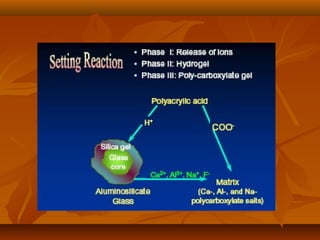
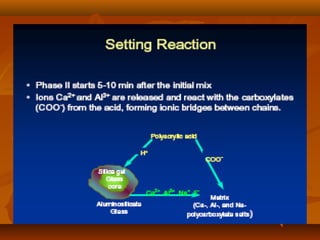
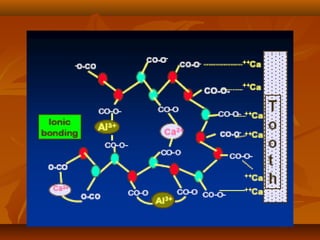
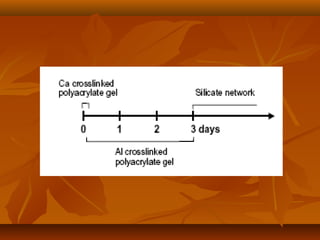
- The setting reaction involves dissolution of the glass powder, precipitation of salts, and hydration of
































![Polyelectrolytes –Polyelectrolytes –
as the name implies are bothas the name implies are both electrolytes and polymers.electrolytes and polymers.
The more important carboxylic acids in the ionomer systemThe more important carboxylic acids in the ionomer system
includeinclude
ACRYLIC ACID,ACRYLIC ACID,
MALEIC ACIDMALEIC ACID
ITACONIC ACIDITACONIC ACID
either in the form of aeither in the form of a conc. aqueous solution (40% to 50% byconc. aqueous solution (40% to 50% by
mass)mass) or blended dry with either water or an aqueous solutionor blended dry with either water or an aqueous solution
of tartaric acidof tartaric acid. [Mclean et al, 1984]. [Mclean et al, 1984]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-46-320.jpg)
![ WaterWater - the REACTION MEDIUM and also plays a role in- the REACTION MEDIUM and also plays a role in
hydrating reaction products.hydrating reaction products.
Tartaric acidTartaric acid
DECREASES viscosity & DELAYING gelationDECREASES viscosity & DELAYING gelation
greater the concentration greater is the delay.greater the concentration greater is the delay.
[Cook, 1983; Hill and Wilson; 1986].[Cook, 1983; Hill and Wilson; 1986].
Prolongs W.T of mixProlongs W.T of mix
Decrase S.TDecrase S.T
INCREASES handling charactersticsINCREASES handling characterstics
MOAMOA ::
Temporary suppresionTemporary suppresion of ionization of polyacidof ionization of polyacid
Enhances extraction of Al fromEnhances extraction of Al from Glass.Glass.](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-47-320.jpg)





![Water present in set cement can be classified
into two forms:
(1) Loosely bound water (readily removed by
dessication)
(2) Tightly bound water (Cannot be removed)
[Wilson and Crisp, 1975; Elliot et
al,1975; Wilson et al, 1981]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-57-320.jpg)
![ As the cement ages, degree of hydration (ratio ofAs the cement ages, degree of hydration (ratio of
tightly bound to loosely bound water) increases. Thistightly bound to loosely bound water) increases. This
is accompanied by an increase in strength andis accompanied by an increase in strength and
modulus and a decrease in plasticity.modulus and a decrease in plasticity.
[Paddoson and Wilson, 1976; Wilson et al, 1981][Paddoson and Wilson, 1976; Wilson et al, 1981]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-58-320.jpg)

![ When subjected to moisture : Absorption ofWhen subjected to moisture : Absorption of
waterwater
(1) Disruption of surface by swelling(1) Disruption of surface by swelling
(2) Loss of substance to oral environment(2) Loss of substance to oral environment
[Wilson et al, 1981;[Wilson et al, 1981;
Causton, 1981; Roulet and Walti, 1984; Phillips andCauston, 1981; Roulet and Walti, 1984; Phillips and
Bishop; 1984]Bishop; 1984]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-60-320.jpg)

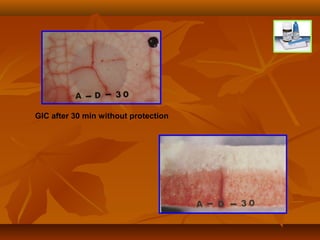
![ MOST EFFECTIVE MATERIALS FORMOST EFFECTIVE MATERIALS FOR
PROTECTING GICSPROTECTING GICS
Low viscosity, light curing bonding agentsLow viscosity, light curing bonding agents..
Proprietary varnishesProprietary varnishes supplied by manufacturerssupplied by manufacturers
Wax / petroleum jellyWax / petroleum jelly
only afford brief protectiononly afford brief protection
(as they are Easily Washed(as they are Easily Washed
Off by tongue movements)Off by tongue movements)
[Earl & Coworkers, 1985][Earl & Coworkers, 1985]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-62-320.jpg)




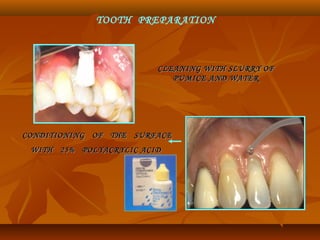

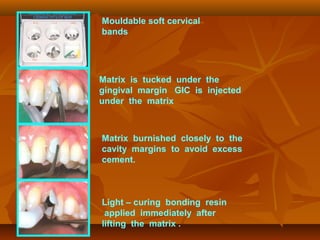
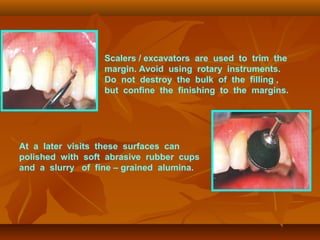
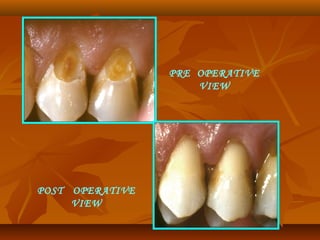



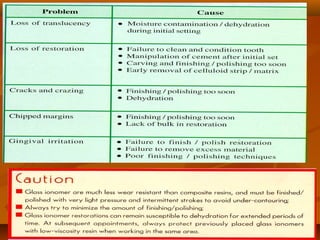



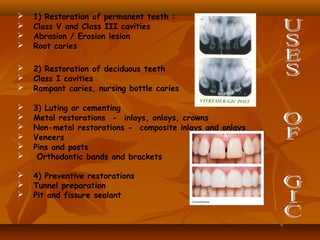
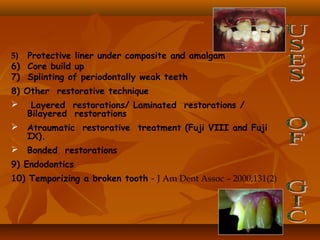
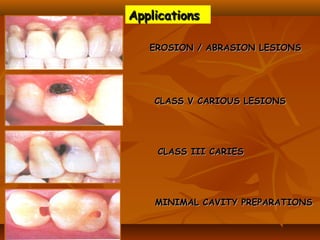


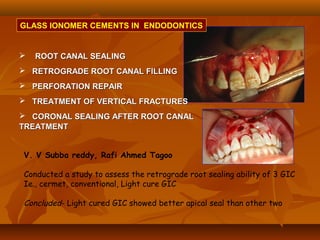
![(1). Ketac-endo strengthens endodontically treated roots(1). Ketac-endo strengthens endodontically treated roots
and may be used for weaker roots which are likely toand may be used for weaker roots which are likely to
be susceptible to vertical root #.be susceptible to vertical root #.
[J. Endod; 2002; Mar ; 28 (3); 217-9][J. Endod; 2002; Mar ; 28 (3); 217-9]
(2) While comparing apical leakage between ketac endo(2) While comparing apical leakage between ketac endo
and grossman sealer, the best results were noted whenand grossman sealer, the best results were noted when
ketac endo sealers were used with lateralketac endo sealers were used with lateral
condensation.condensation.
[Oral Surg oral Med Oral Pathol; 1994 : Dec; 78)[Oral Surg oral Med Oral Pathol; 1994 : Dec; 78)](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-90-320.jpg)
![(3) Vitrebond has a potential as root-end filling material as the(3) Vitrebond has a potential as root-end filling material as the
tissue response is more favourable than that to amalgamtissue response is more favourable than that to amalgam ..
[ Int. Endod J. 1997, Mar, 30 (2); 102-14][ Int. Endod J. 1997, Mar, 30 (2); 102-14]
(4) Teeth without intraorifice barrier leaked significantly more(4) Teeth without intraorifice barrier leaked significantly more
than teeth with vitrebond intraorifice barriersthan teeth with vitrebond intraorifice barriers..
[J. Endod; 1999 Sep; 25(9)][J. Endod; 1999 Sep; 25(9)]
(5) MTA alone or vitremer in combination with collagen(5) MTA alone or vitremer in combination with collagen
sponge can be used effectively in the treatment ofsponge can be used effectively in the treatment of
perforations in a furcation areaperforations in a furcation area..
[Int. Dent. J. ; 2005 Jun; 55 (3)][Int. Dent. J. ; 2005 Jun; 55 (3)]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-91-320.jpg)







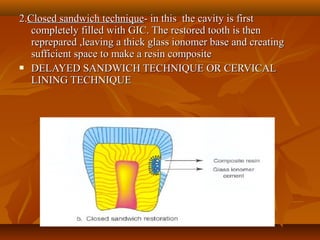
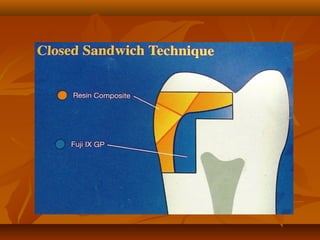















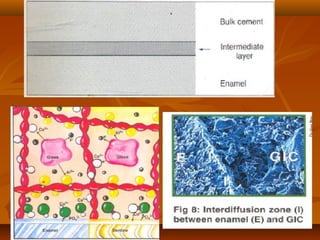
![MECHANISM OF ADHESION TO ENAMEL & DENTINMECHANISM OF ADHESION TO ENAMEL & DENTIN
Bonding of GIC to tooth structure - CHEMICAL one and not aBonding of GIC to tooth structure - CHEMICAL one and not a
MECHANICAL one.MECHANICAL one.
The PRINCIPAL mode of adhesion being BONDING TOThe PRINCIPAL mode of adhesion being BONDING TO
HYDROXYAPATITEHYDROXYAPATITE
VARIOUS THEORIES PROPOSEDVARIOUS THEORIES PROPOSED ::
(1)(1) Chelation of Ca2+ contained in hydroxyapatite isChelation of Ca2+ contained in hydroxyapatite is
responsible for adhesion.responsible for adhesion.
[Smith, 1968].[Smith, 1968].
(2) Hydroxyapatite + Polyacrylic acid(2) Hydroxyapatite + Polyacrylic acid POLYACRYLATE IONSPOLYACRYLATE IONS
made strong ionic bonds with Ca2+ of hydroxyapatite in enamel andmade strong ionic bonds with Ca2+ of hydroxyapatite in enamel and
dentin.dentin. [Beech, 1973][Beech, 1973]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-120-320.jpg)







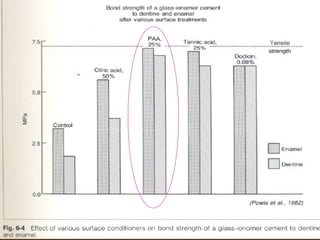

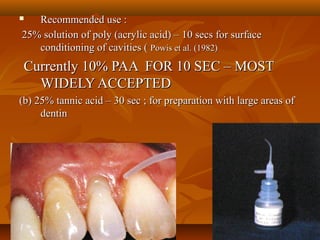









![FLUORIDE RELEASE ANDFLUORIDE RELEASE AND
RECHARGEABILITRECHARGEABILITYY
GIC have a cariostatic action.GIC have a cariostatic action.
spread of caries is arrested at restoration / cavity wall marginspread of caries is arrested at restoration / cavity wall margin
F- originates from the fluoride used in preparingF- originates from the fluoride used in preparing
aluminosilicate glasses (CaF, Na3AlF6aluminosilicate glasses (CaF, Na3AlF6) [Kent et al 1976]) [Kent et al 1976]
This F- is released as NaF [Wilson and Coworkers, 1985]This F- is released as NaF [Wilson and Coworkers, 1985]
and also as CaF2and also as CaF2
[Powis and Wilson, 1987[Powis and Wilson, 1987](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-141-320.jpg)
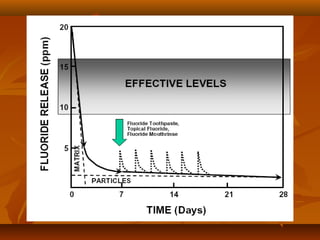
![ F- is mainly leached as a sodium salt, which is not aF- is mainly leached as a sodium salt, which is not a
matrix-forming species. Thus, loss of F- does notmatrix-forming species. Thus, loss of F- does not
weaken the cementweaken the cement
F- is released by DIFFUSION MECHANISM whereF- is released by DIFFUSION MECHANISM where
the rate of release is proportional to the square root ofthe rate of release is proportional to the square root of
timetime ..[Wilson et al 1985; Kuhn and Wilson, 1985][Wilson et al 1985; Kuhn and Wilson, 1985]
is released for a sustained period of time – at leastis released for a sustained period of time – at least
18 months.18 months.
Thickly mixed cements release more F- than thinlyThickly mixed cements release more F- than thinly
mixedmixed. [Meryon and Smith, 1984]. [Meryon and Smith, 1984]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-142-320.jpg)
![ MECHANISM OF ACTION OF F-MECHANISM OF ACTION OF F-
(1) F- is taken up by enamel apatite to form(1) F- is taken up by enamel apatite to form
flurohydroxyapatite at enamel surface. Thisflurohydroxyapatite at enamel surface. This ↑↑ resistance toresistance to
plaque acidsplaque acids. [Brundevold et al, 1967; McLundie and Murray, 1972; Muhler. [Brundevold et al, 1967; McLundie and Murray, 1972; Muhler
1956; Moreno et al, 1977].1956; Moreno et al, 1977].](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-143-320.jpg)
![ 2) F-2) F- ↓↓ surface energy of apatite therebysurface energy of apatite thereby ↓↓ thethe
adherence of dental plaque to tooth surface.adherence of dental plaque to tooth surface.
[Glantz 1969][Glantz 1969]
(3) F- aids in remineralisation of damaged enamel(3) F- aids in remineralisation of damaged enamel
[Silverstone, 1978][Silverstone, 1978]
(4) F- changes the composition of bacterial plaque(4) F- changes the composition of bacterial plaque
which alters the carbohydrate metabolism of dentalwhich alters the carbohydrate metabolism of dental
plaqueplaque [Horowitz et al, 1977; Silverstone, 1978; Mellberg, 1977;[Horowitz et al, 1977; Silverstone, 1978; Mellberg, 1977;
Ingram and Nash, 1980].Ingram and Nash, 1980].
A fluoride enriched tooth surface has a critical pH ofA fluoride enriched tooth surface has a critical pH of
4.5.4.5.](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-144-320.jpg)










![BIOCOMPATIBILITYBIOCOMPATIBILITY
Freshly mixed- very acidic (.9 -1.6)Freshly mixed- very acidic (.9 -1.6)
Ph rises rapidly with in first 20 minPh rises rapidly with in first 20 min
EFFECT ON PULP AND CELLS :EFFECT ON PULP AND CELLS :
Freshly mixedFreshly mixed GICs - cytotoxicGICs - cytotoxic
[Dahl and Tronstad, 1976; Meryon and Coworkers, 1983].[Dahl and Tronstad, 1976; Meryon and Coworkers, 1983].
Although freshly mixed GICs inhibited cellularAlthough freshly mixed GICs inhibited cellular
proliferation, it was not cytotoxic.proliferation, it was not cytotoxic.
[Kawahara and cowkers, 1979][Kawahara and cowkers, 1979]
GICs cause greater inflammatory response than Zn-EGICs cause greater inflammatory response than Zn-E
cement but less than ZnPO4 cement and related dentalcement but less than ZnPO4 cement and related dental
silicate cements.silicate cements. [Plant et al, 1984][Plant et al, 1984]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-156-320.jpg)


![ Citric acid when used as SURFACE CONDITIONERCitric acid when used as SURFACE CONDITIONER
on cut tubules causeson cut tubules causes ↑↑ inflammatory response.inflammatory response.
[Cotton & Siegel, 1978][Cotton & Siegel, 1978]
Linings of ZnOE or Ca(OH)2 cement are requiredLinings of ZnOE or Ca(OH)2 cement are required
where < 1 mm of sound dentin remains over the pulp.where < 1 mm of sound dentin remains over the pulp.
[McLean and Wilson, 1978][McLean and Wilson, 1978]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-159-320.jpg)
![ FACTORS RESPONSIBLE FORFACTORS RESPONSIBLE FOR
BIOCOMPATIBILITY OF GIC :BIOCOMPATIBILITY OF GIC :
(1) Minimal exotherm on setting(1) Minimal exotherm on setting
(2) Rapid neutralization on mixing powder and liquid(2) Rapid neutralization on mixing powder and liquid
(3) Slow release of ions which are generally beneficial.(3) Slow release of ions which are generally beneficial.
[J. Biomater Sci Polym Ed. 1991; 2(4); 277-85][J. Biomater Sci Polym Ed. 1991; 2(4); 277-85]
On the contrary:On the contrary:
(1) Resin modified GICs (ComPoglass, Fuji II LC,(1) Resin modified GICs (ComPoglass, Fuji II LC,
Protec Cem) and GC lining cements are more toxic toProtec Cem) and GC lining cements are more toxic to
pulp than conventional GIC.pulp than conventional GIC.
RM GICs should not be applied directly onto pulpRM GICs should not be applied directly onto pulp
cells.cells.
[Oper Dent; July / Aug; 28 (4)]; 2003[Oper Dent; July / Aug; 28 (4)]; 2003](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-160-320.jpg)
![(2) The principal compounds responsible for(2) The principal compounds responsible for
cytotoxicity of RMGIC – unpolymerised resincytotoxicity of RMGIC – unpolymerised resin
monomer (HEMA) and for MGIC – Cu2+, Ag+.monomer (HEMA) and for MGIC – Cu2+, Ag+.
[Biomed Mater Res; 48; 277-88; 1999][Biomed Mater Res; 48; 277-88; 1999]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-161-320.jpg)














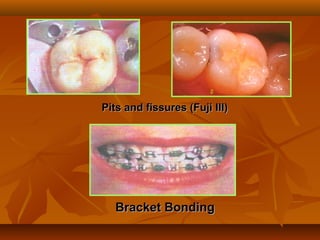
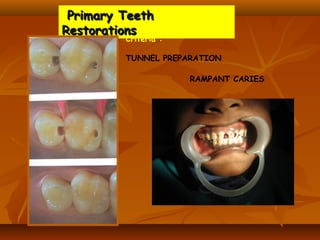
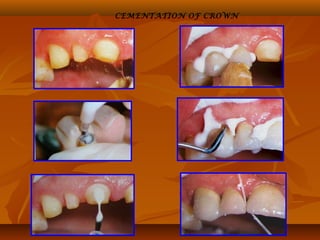
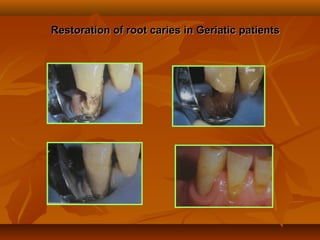
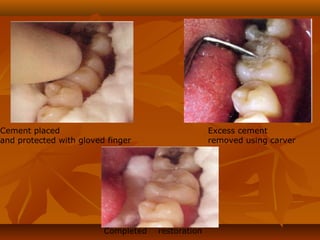
![ Core build up .
Restoration of primary teeth.
Restoration of approximal lesions [ tunnel
preparation ] .
Treatment of root caries
Repair of defective margins in restorations.
Retrograde root fillings.](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-176-320.jpg)

![IN 1988 THE FIRST LIGHT CURABALE GIC WAS INTRODUCEDIN 1988 THE FIRST LIGHT CURABALE GIC WAS INTRODUCED
COMPOSITIONCOMPOSITION
Powder componentPowder component contains ion – leachablecontains ion – leachable
fluoroaluminosilicate glass particles & initiators for light curingfluoroaluminosilicate glass particles & initiators for light curing
& / or chemical curing.& / or chemical curing.
Initiator / ActivatorInitiator / Activator
Liquid componentLiquid component contains water & polyacrylic acid modifiedcontains water & polyacrylic acid modified
with methacrylate & hydroxy ethyl methacrylate [ HEMA ]with methacrylate & hydroxy ethyl methacrylate [ HEMA ]
monomers for polymerization.monomers for polymerization.
Chemical
polymerization
Initiator : Hydrogen peroxide
Activator : Ascorbic acid
Co activator: Cupric sulphate..
light activated
Initiator: Camphoroquinone
Activator: Sodium p toluene sulphinate.
Photo activator: 4-NN dimethyl amino
benzonate.](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-178-320.jpg)
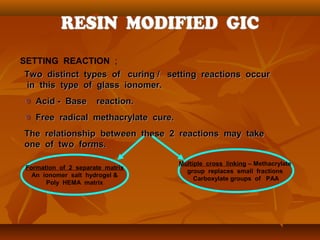





![A . RESIN MATRIX .
1)1) UDMAUDMA
2)2) TCB RESIN [ TETRA CARBOXYLIC ACID + HEMA AS SIDE CHAIN ].TCB RESIN [ TETRA CARBOXYLIC ACID + HEMA AS SIDE CHAIN ].
3)3) FILLERS - STRONTIUM FLUORO SILICATE GLASS & DEHYDRATEDFILLERS - STRONTIUM FLUORO SILICATE GLASS & DEHYDRATED
PAA ACIDPAA ACID
4)4) STRONTIUM FLUORIDE..STRONTIUM FLUORIDE..
B . PRIMER / ADHESIVE SYSTEM.
1)1) PENTA - This is an acidic monomer made up of phosphoricPENTA - This is an acidic monomer made up of phosphoric
acidacid
2)2) TEGDMA - Provides elasticity to the cured primer / adhesive.TEGDMA - Provides elasticity to the cured primer / adhesive.
3)3) ACETONE - Acts as a solvent which carries the resin & helpsACETONE - Acts as a solvent which carries the resin & helps
to wet the tooth surface & assist the penetration of resin into wet the tooth surface & assist the penetration of resin in
the dentin surface.the dentin surface.](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-185-320.jpg)







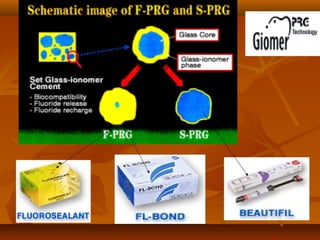

![STUDIES :STUDIES :
(1) Release F- but do not have an initial “burst” type of(1) Release F- but do not have an initial “burst” type of
release like GI and long term (28 days) release isrelease like GI and long term (28 days) release is
decreasedecrease
[Oper Dent : 2002; 27 ; 259-65][Oper Dent : 2002; 27 ; 259-65]
(2) When polished with sof-lex disks, they have a(2) When polished with sof-lex disks, they have a
smoother surface than a GI. Comparable to compomersmoother surface than a GI. Comparable to compomer
and resin composite.and resin composite.
[Oper Dent; 2002; 27 ; 161-66][Oper Dent; 2002; 27 ; 161-66]](https://image.slidesharecdn.com/glassionomercement-170610024451/85/Glass-ionomer-cement-195-320.jpg)