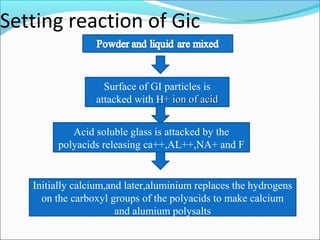
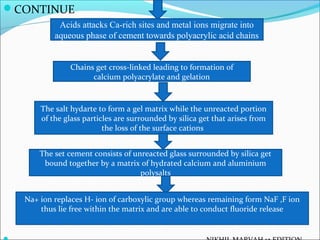
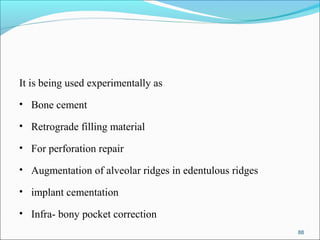
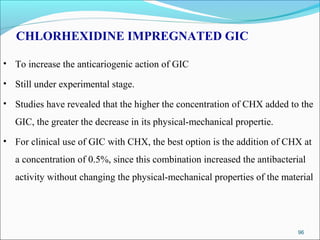
This document provides an overview of glass ionomer cement (GIC), including:
1. The history and development of GIC from its invention in 1972 to current modifications.
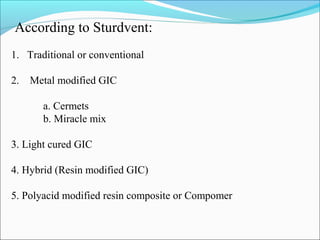
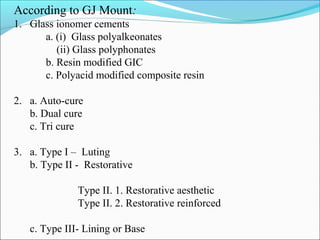
2. Classifications of GIC based on various criteria such as type, clinical use, and curing method.
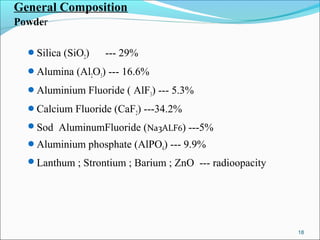
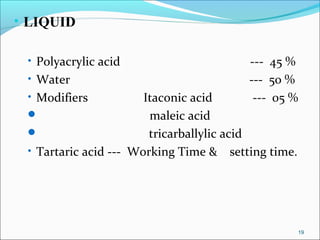
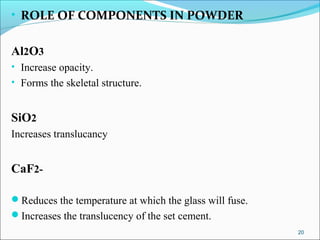
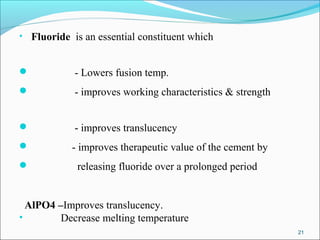
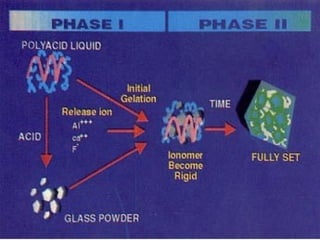
3. The composition of GIC including glass powder, polyacrylic acid liquid, and their roles in the setting reaction.
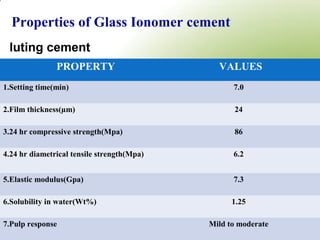
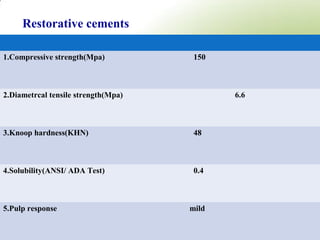
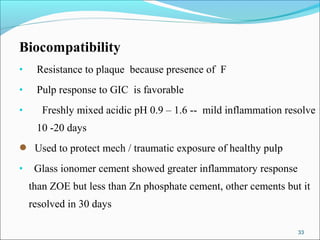
4. Key properties of GIC such as working time, strength, fluoride release, biocompatibility, and indications/contraindications for use.
5. Modifications to traditional GIC including water-hardening and metal-modified versions.