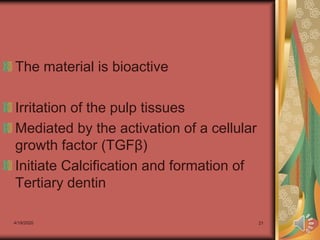
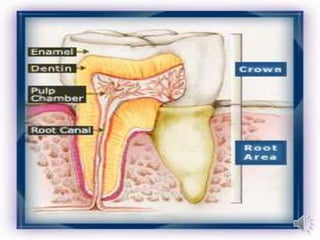
Calcium hydroxide cements were introduced by Hermann in 1920 as an alternative to viewing exposed pulps as "doomed organs." Calcium hydroxide cements promote healing in clinical situations by creating an alkaline environment. They are formed through a reaction of calcium oxide with water to create calcium hydroxide. Typical calcium hydroxide cements are composed of calcium hydroxide, zinc oxide, zinc stearate, and ethyl toluene sulphonamide. They set through exothermic chemical reactions, have biocompatibility that can destroy bacteria and initiate reparative dentin formation, but have low strength and solubility in moisture.