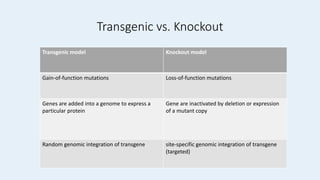
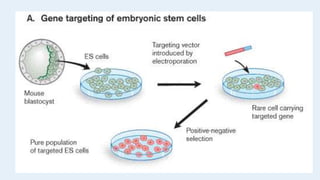
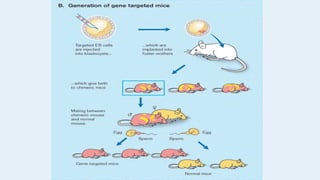
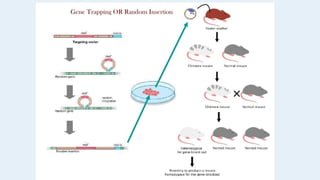
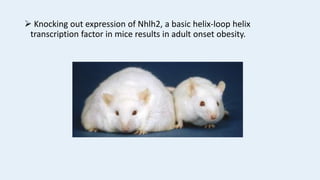
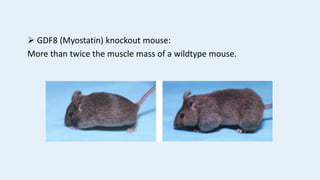
Gene knockout animals are genetically engineered organisms with inactivated genes. Mice are commonly used for knockout experiments due to their close genetic similarity to humans and low cost of breeding. Knocking out genes in mice provides insights into gene function and human disease modeling. The gene targeting method uses embryonic stem cells to precisely knockout a gene, while gene trapping does not require known gene sequences but confirmation of knockout is needed. Knockout studies have furthered understanding of cancer, obesity, and other diseases.