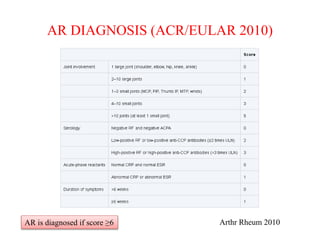
This document discusses rheumatoid arthritis (RA) and the evolution of drugs used to treat it. RA is a chronic inflammatory disease that causes joint damage and disability. While the cause is unknown, it involves an immune system response leading to inflammation. Treatment has progressed from nonsteroidal anti-inflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs) like methotrexate, to biological DMARDs that target specific cytokines and cells involved in the immune response, such as tumor necrosis factor (TNF) inhibitors and interleukin-6 (IL-6) inhibitors. Larger clinical trials were required to develop these targeted biologic therapies compared to earlier drugs. Animal models of collagen