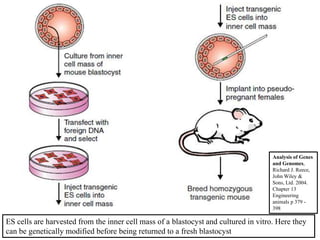
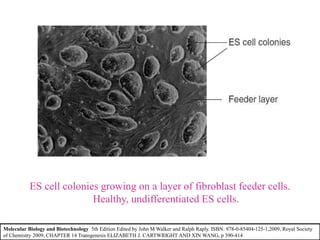
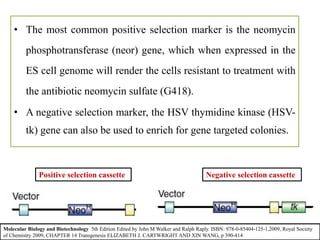
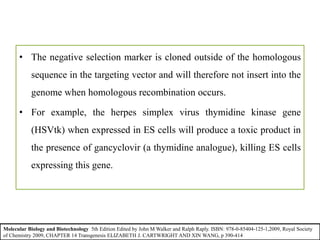
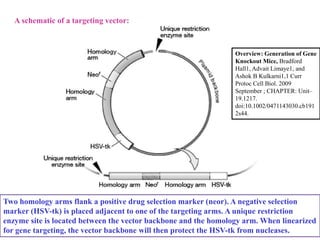
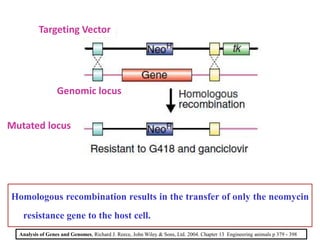
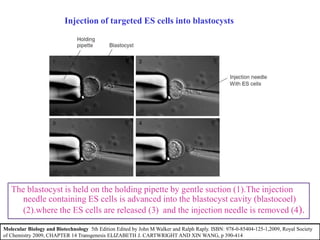
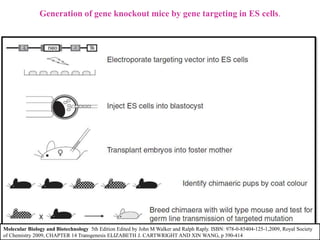
Gene knock out technology uses embryonic stem cells to introduce targeted mutations into the mouse genome, allowing the study of gene function. A targeting vector containing the mutated gene sequence flanked by homologous DNA is introduced into embryonic stem cells. Cells with the mutation incorporated via homologous recombination are identified and injected into blastocysts, generating chimeric mice. Breeding of these mice can produce strains lacking the gene of interest, enabling investigation into the effects of its absence. This technology was developed in the 1980s-1990s and its creators were awarded the 2007 Nobel Prize in Physiology or Medicine.