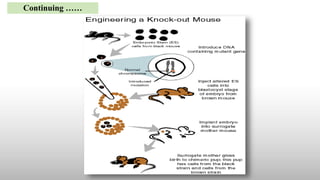
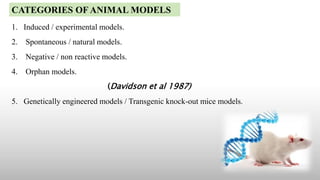
The document discusses animal models, specifically knockout mice, which are mice that have had a specific gene inactivated through replacement or disruption. It provides definitions of animal models and describes their importance in studying diseases and testing drugs before human trials. Knockout mice are created through a process of isolating a target gene, engineering a nonfunctional version, inserting it into stem cells which are then implanted in mouse embryos. This generates mice lacking the gene's function, allowing study of its role. While valuable, limitations include some genes being lethal to remove and variability in the procedure.
![Developments of Animal Model [Knockout Mice].
Presented by
Anil Behera
Subject – Animal Biotechnology](https://image.slidesharecdn.com/developmentofanimalmodels-anil-200804084020/75/Development-of-animal-model-Knockout-Mice-1-2048.jpg)

![1. Small rodents [ ex-mice, rats, hamsters, minks.]
2. Larger animals [ex- dogs and sheep.]
3. Non-human primates [ex-baboon, macaque, chimpanzee and gorilla.]
4. Others [ex-apes, cats, horses, guinea pigs, mongooses, wolves, foxes, rabbits, ferret etc.]
CLASSIFICATION OF ANIMAL MODELS](https://image.slidesharecdn.com/developmentofanimalmodels-anil-200804084020/85/Development-of-animal-model-Knockout-Mice-5-320.jpg)
![CHOICE OF ANIMALS [CRITERIA FOR SPECIES SELECTION]
• Availability of laboratory facilities
• Presence of a breeding colony
• Cost
• Ease of handling
• Ease of housing
• Relatedness to humans
• Limitations imposed by the size of body structures
• Availability of appropriately sized research instrument
• Transferability of information
• Ethical implications.
etc.](https://image.slidesharecdn.com/developmentofanimalmodels-anil-200804084020/85/Development-of-animal-model-Knockout-Mice-6-320.jpg)