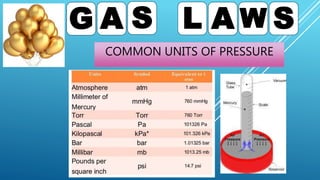
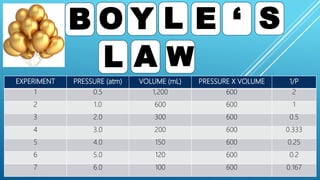
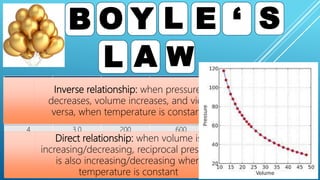
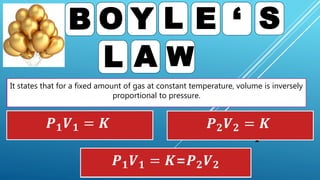
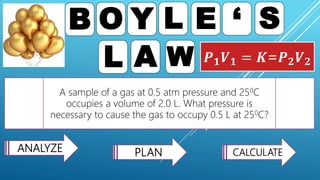
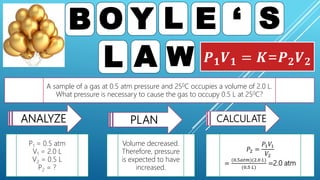
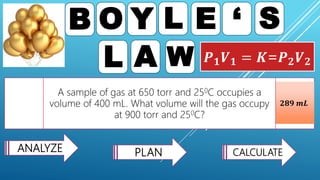
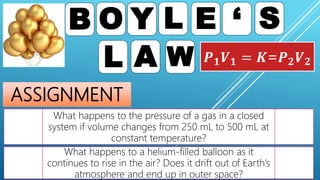
This document discusses gas laws, including Boyle's law. It provides examples showing that for a fixed amount of gas at constant temperature, volume and pressure are inversely proportional. Specifically, it states that Boyle's law describes how doubling the pressure halves the volume of a gas, and halving the pressure doubles the volume, at constant temperature. It also provides sample problems and solutions demonstrating how to use Boyle's law equations to calculate changes in pressure or volume given one is fixed.