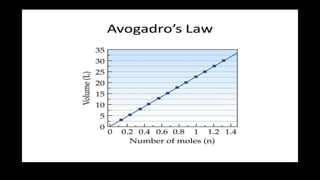
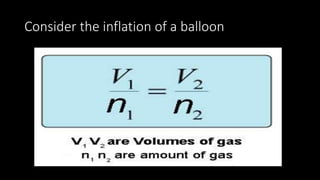
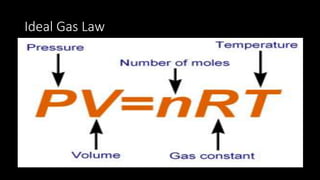
1. The document discusses the fundamental properties and laws governing gases, including pressure, volume, temperature, amount of gas, and how they relate based on Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, and the ideal gas law.
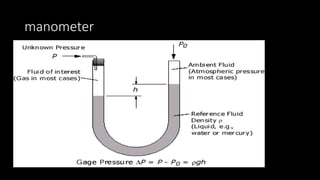
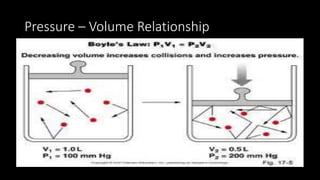
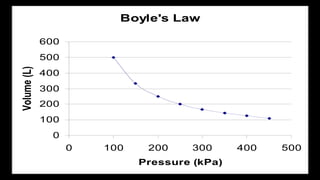
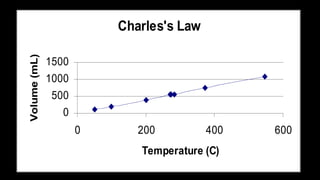
2. Key concepts covered include the definition of pressure, different pressure units, relationships between pressure and volume, relationships between temperature and volume, and how the number of gas molecules affects volume.
3. Examples are provided to demonstrate how to use the gas laws to calculate pressure, volume, temperature, or amount of gas under different conditions.