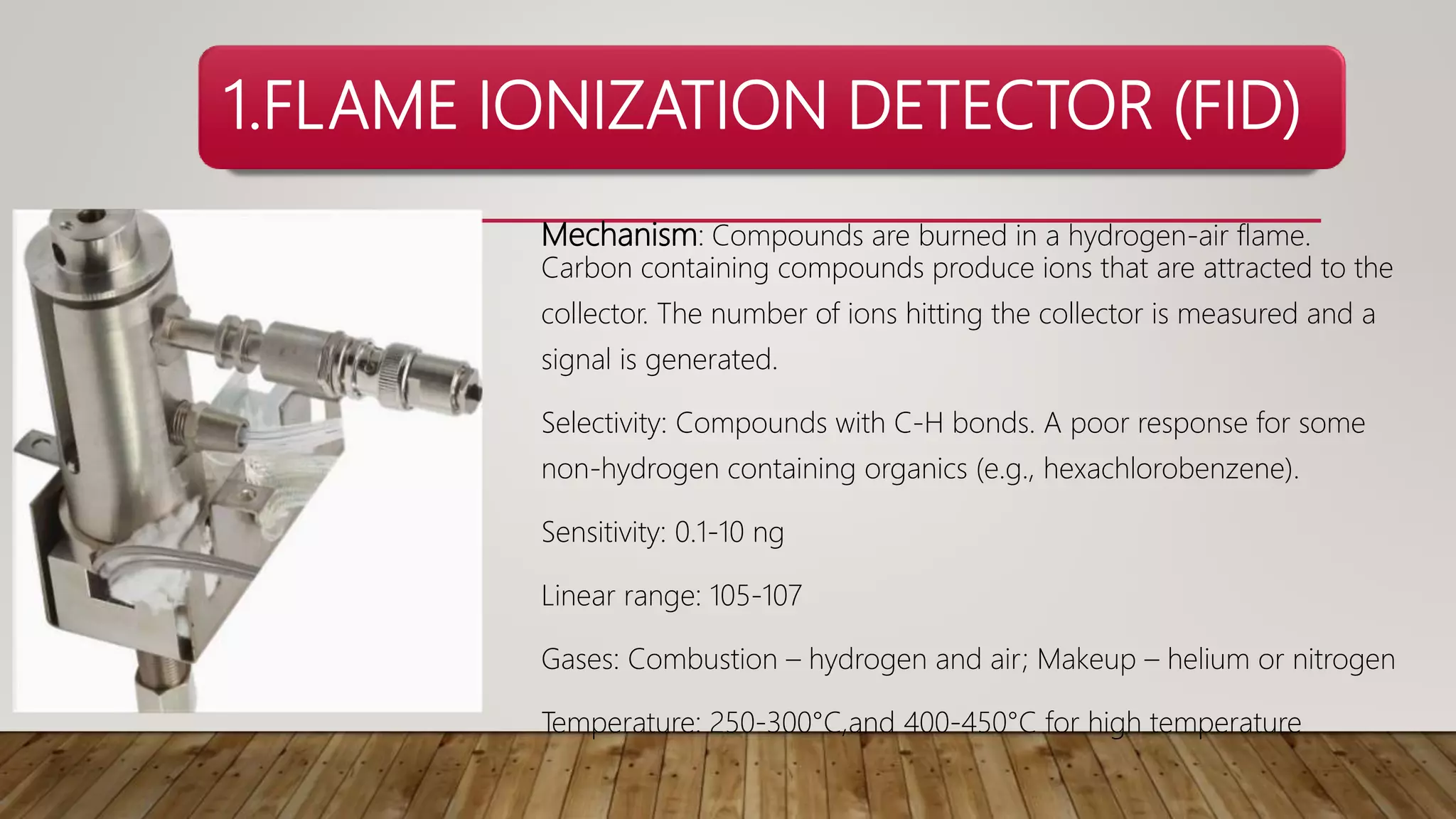
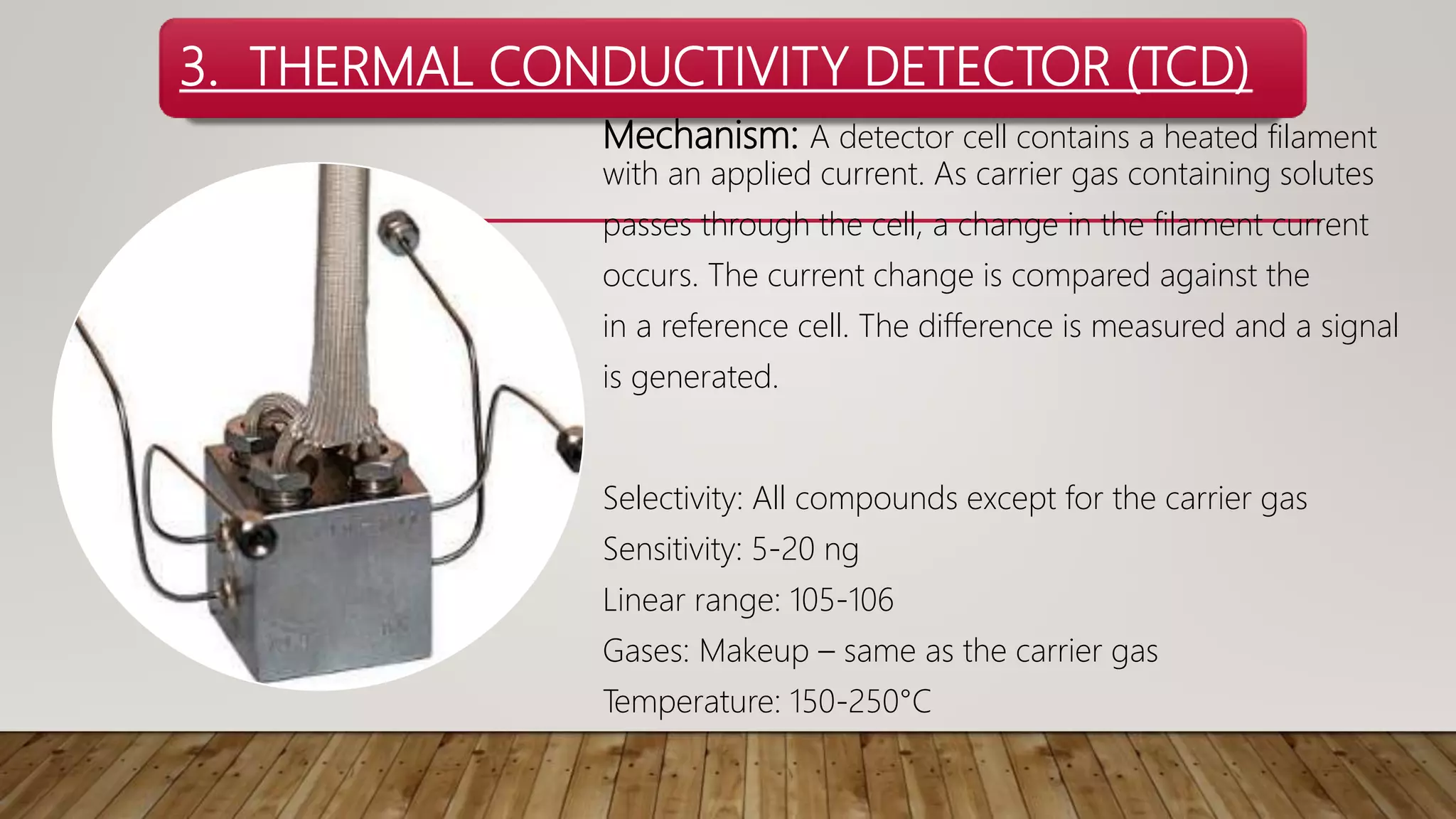
Chromatography is a laboratory method for separating mixtures using a mobile phase and stationary phase. Various detectors are employed in chromatography, including the Flame Ionization Detector (FID), Nitrogen Phosphorus Detector (NPD), Thermal Conductivity Detector (TCD), Electrolytic Conductivity Detector (ELCD), and Photoionization Detector (PID), each with distinct mechanisms and selectivity for different compounds. The document provides detailed characteristics, mechanisms, sensitivities, and operational parameters for each type of detector.