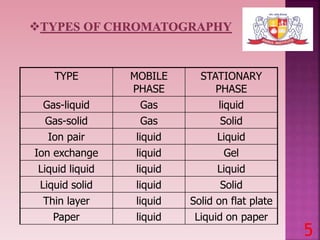
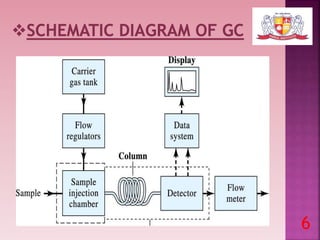
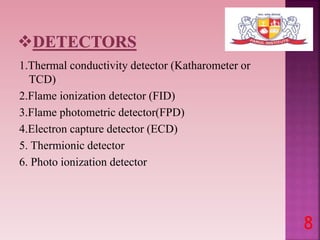
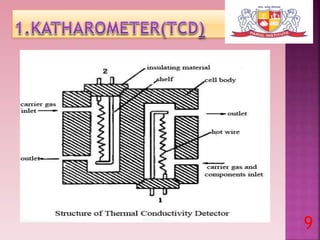
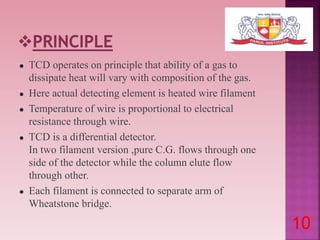
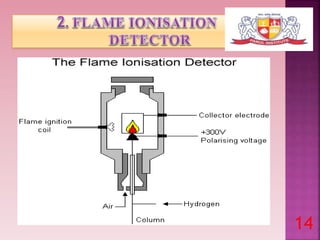
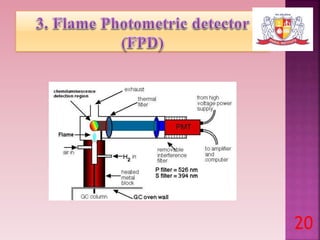
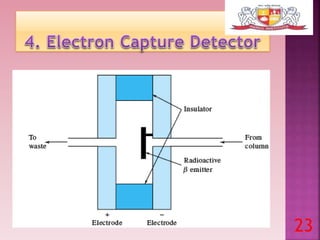
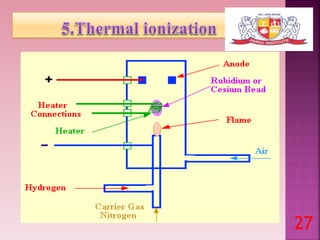
This document discusses gas chromatography and its detectors. It begins by explaining that gas chromatography is a separation technique that uses a mobile gas phase and a stationary liquid or solid phase. The mobile phase is a carrier gas and the stationary phase can be solid or liquid coated on a support. It then describes different types of gas chromatography based on the stationary phase used. The document outlines the basic components and process of gas chromatography. It discusses the different types of detectors used in gas chromatography including thermal conductivity, flame ionization, flame photometric, electron capture, photoionization and others. It provides details on the principles and applications of each detector type.