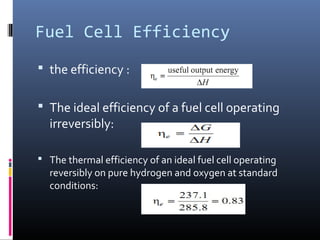
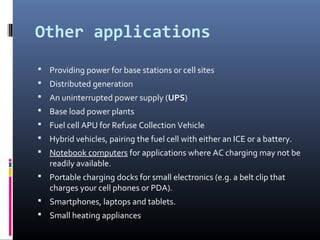
The document discusses fuel cells, which are devices that convert chemical energy from fuels like hydrogen into electricity with minimal air pollution emissions. It details various types of fuel cells, particularly the polymer electrolyte membrane fuel cell (PEMFC), including their reactions, thermodynamic principles, and efficiency. The document also outlines applications of fuel cells in power systems and portable electronics.








![Electrochemical Aspects
-The standard free energy change of the fuel cell reaction is
indicated by the equation :
∆G = –nFE-
The value of ∆G corresponding :
*∆G= −229 kJ/mol,
*n = 2,
*F = 96500 C/g.mole electron,
⇒ E = 1.229 V.
The enthalpy change ∆H for a fuel cell reaction:
∆H = –nFEt
-Nernst equation :
E = E0+ (RT/2F) ln [PH2/PH2O] + (RT/2F) ln [PO2 1/2]](https://image.slidesharecdn.com/fuelcellpresentation-120930210434-phpapp01/85/Fuel-cell-9-320.jpg)