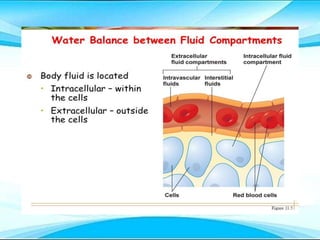
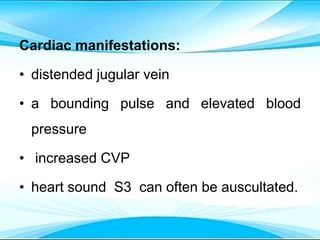
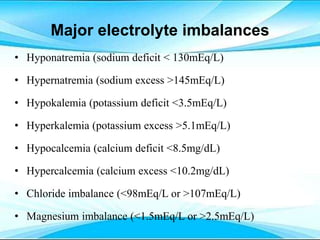
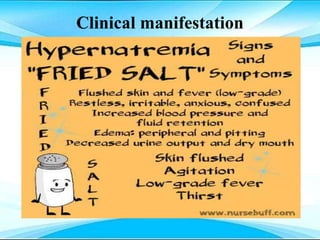
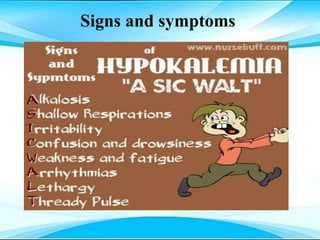
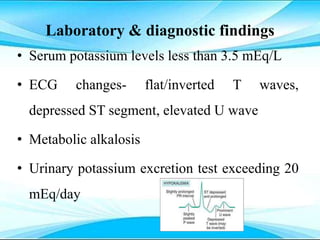
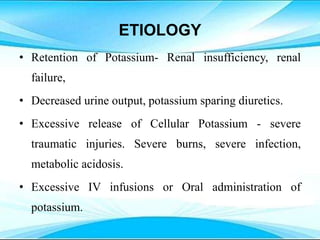
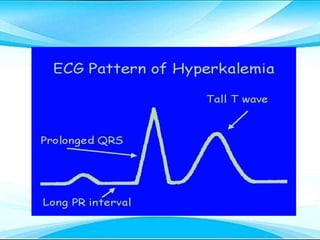
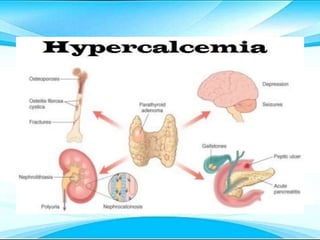
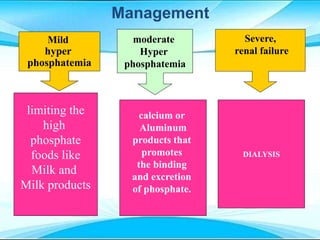
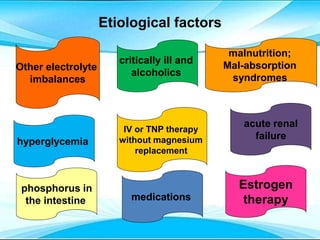
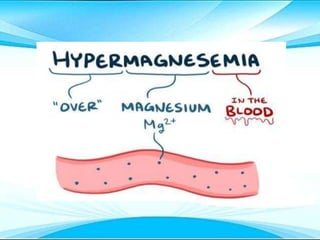
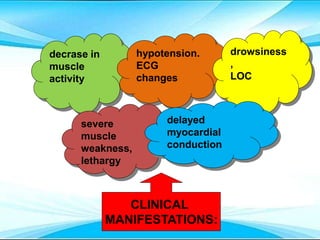
This document discusses fluid and electrolyte imbalances. It begins by explaining that water makes up 60% of the adult body weight and is divided between intracellular and extracellular fluid. The five major types of fluid imbalances are then defined as extracellular fluid volume deficit, intracellular fluid volume deficit, extracellular fluid volume excess, intracellular fluid volume excess, and extracellular fluid volume shift. Causes, signs and symptoms, and treatment approaches are provided for each type of imbalance. Common electrolyte imbalances like hyponatremia, hypernatremia, and hypokalemia are also explained.