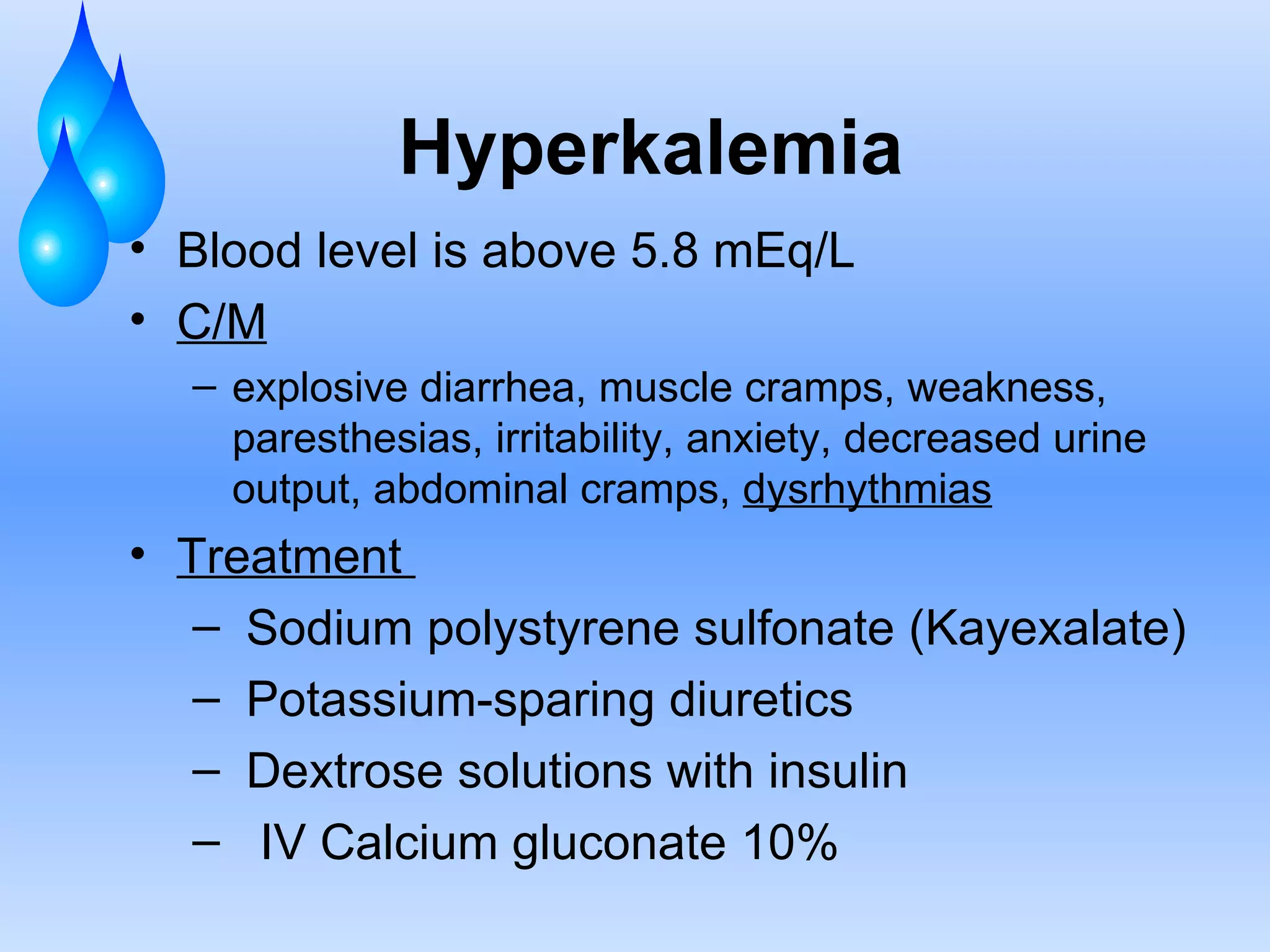
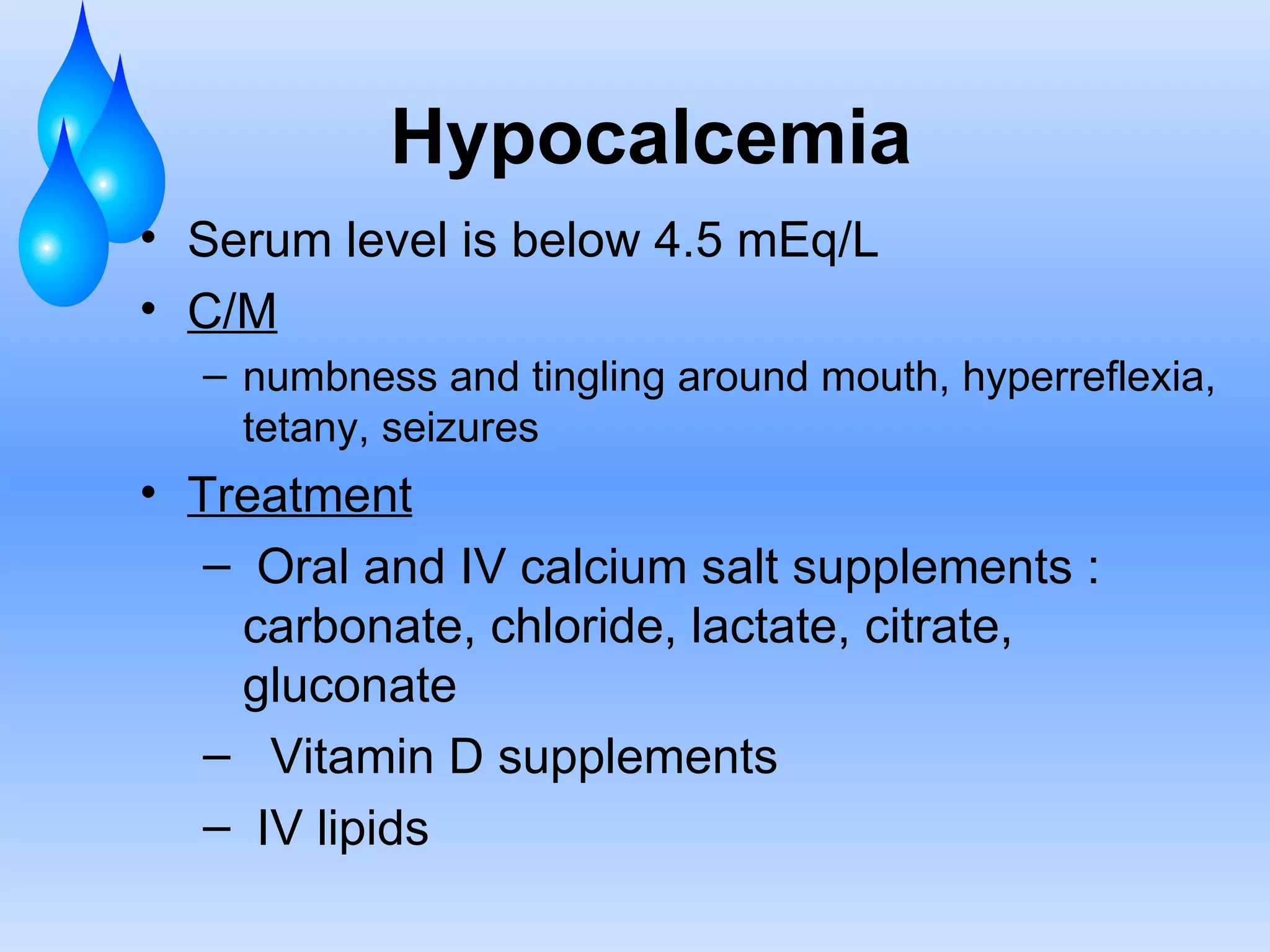
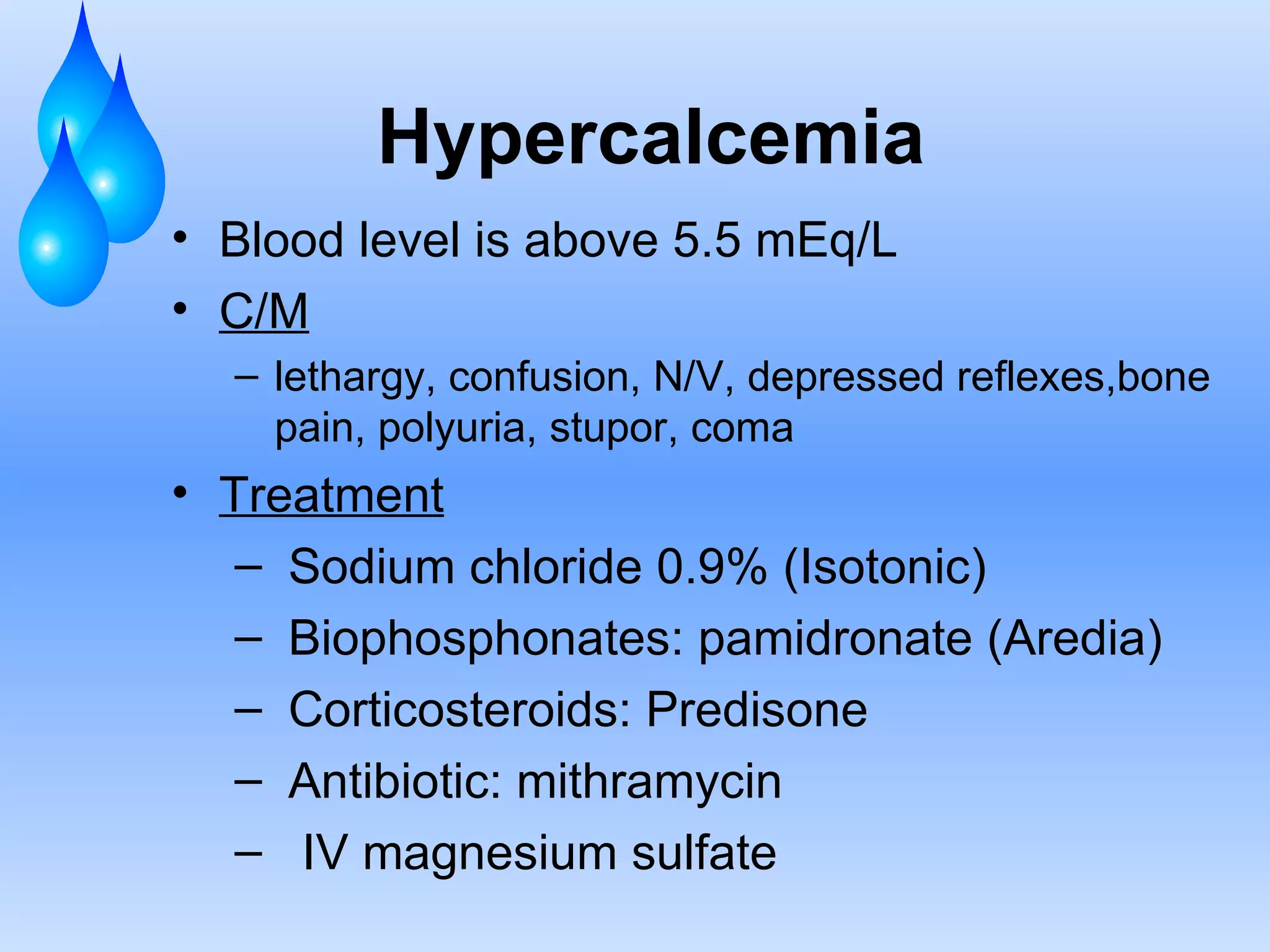
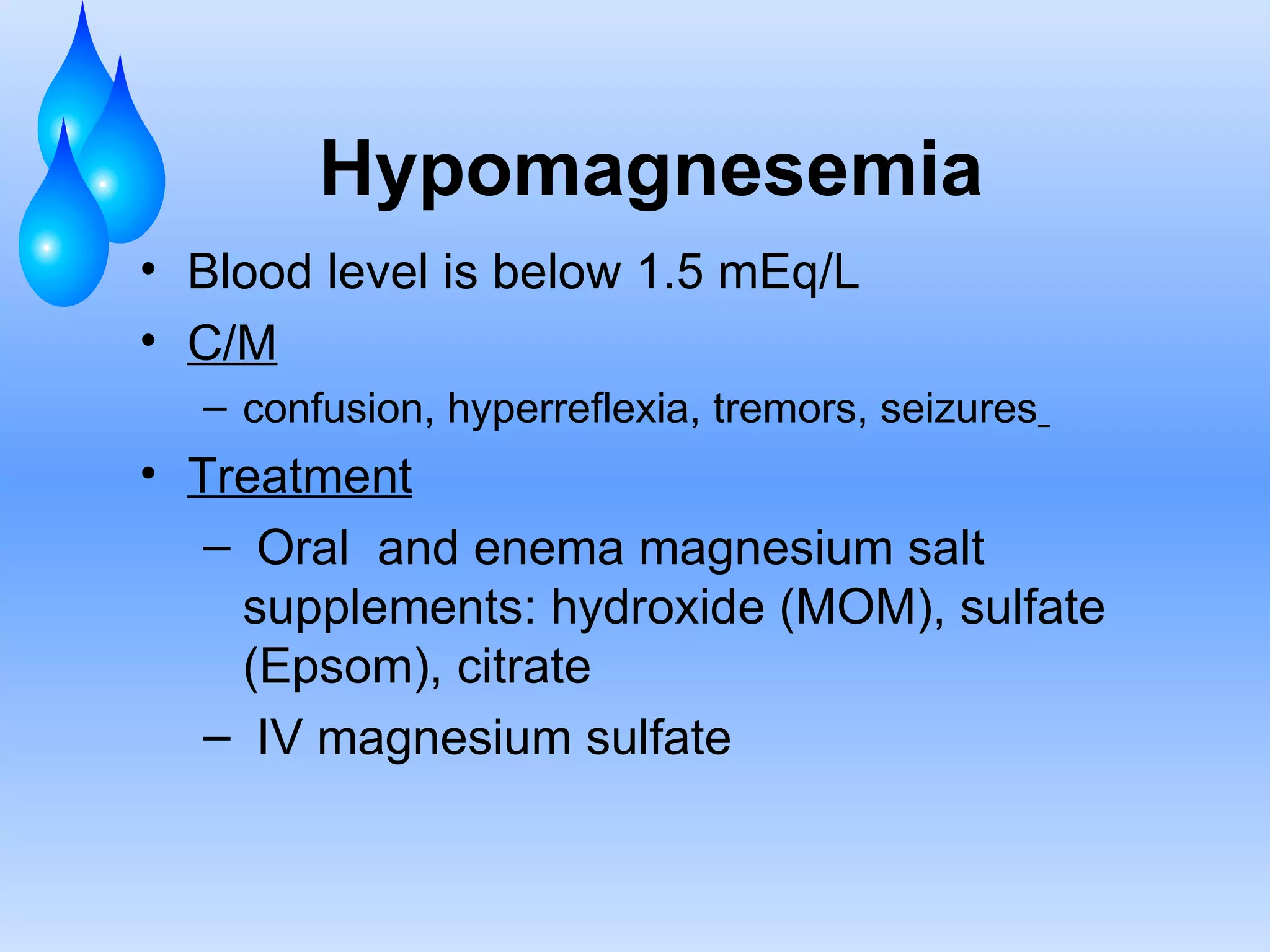
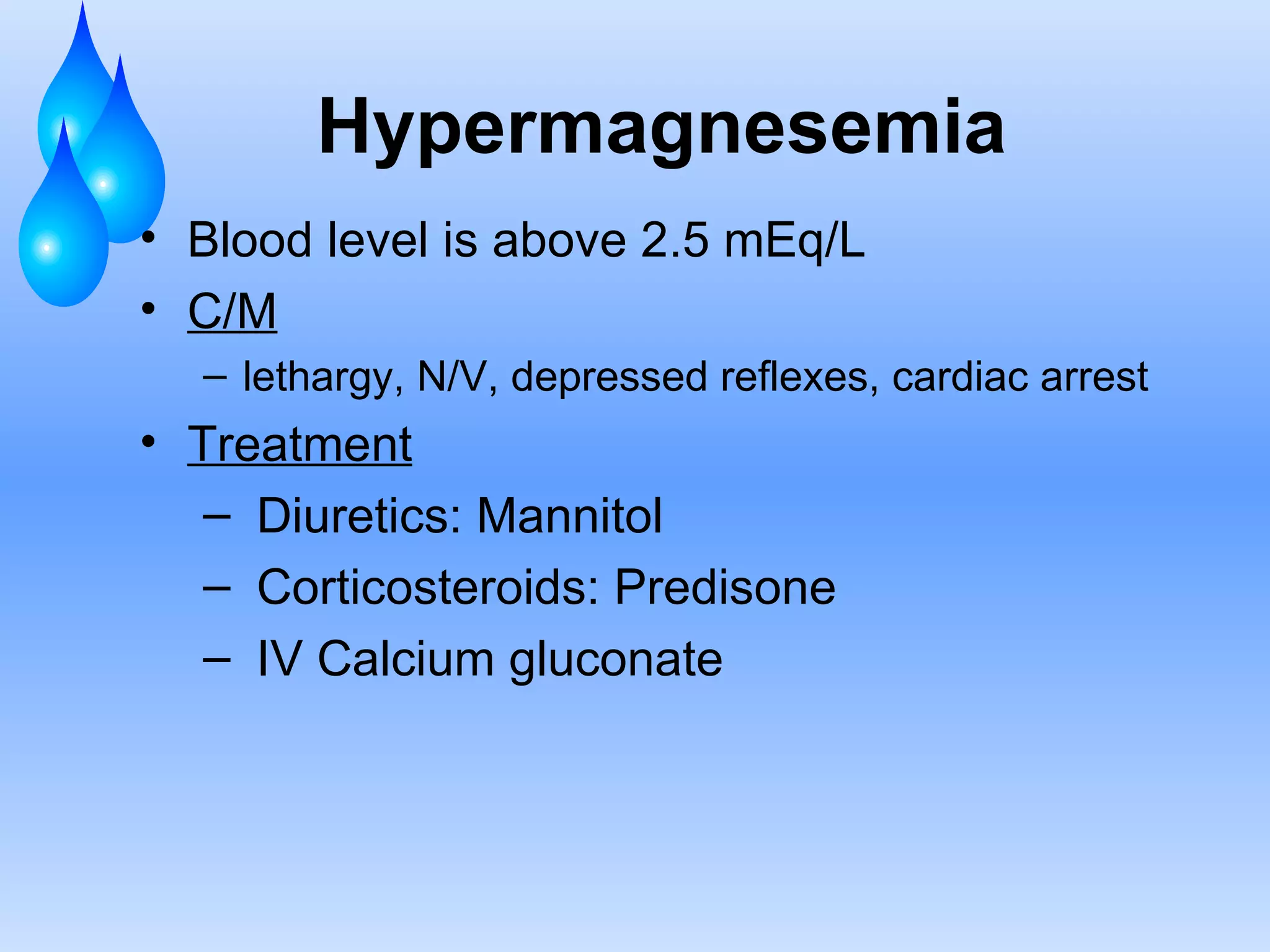
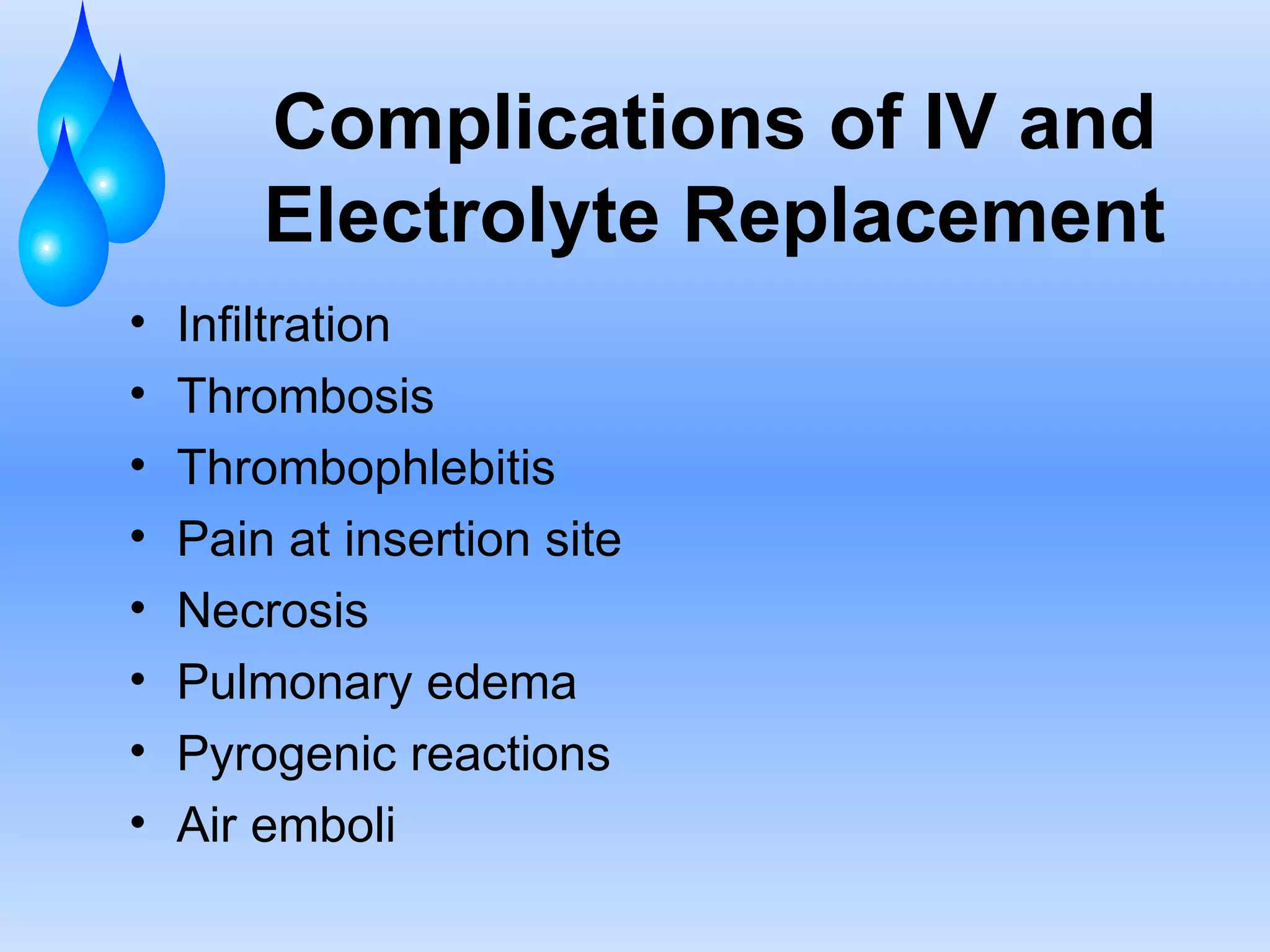
This document discusses fluid and electrolyte balance and replacement. It covers the body's fluid compartments, composition of body fluids including electrolytes and solutes, kidney function in fluid regulation, hormonal control of fluid balance, causes of fluid imbalances, diagnostic tests, and electrolyte abnormalities including sodium, potassium, calcium and magnesium imbalances. Treatment approaches are provided for restoring normal fluid and electrolyte levels.