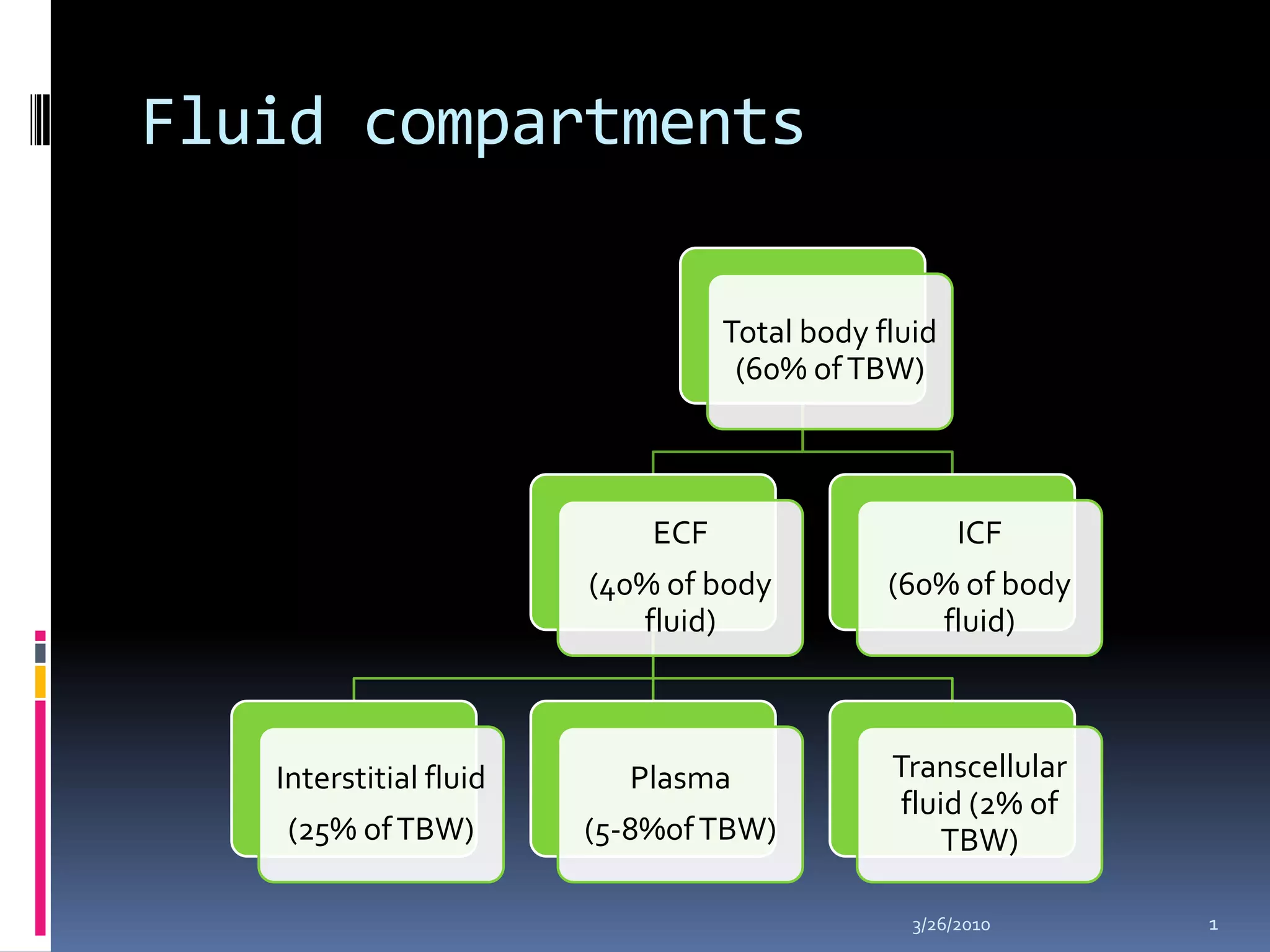
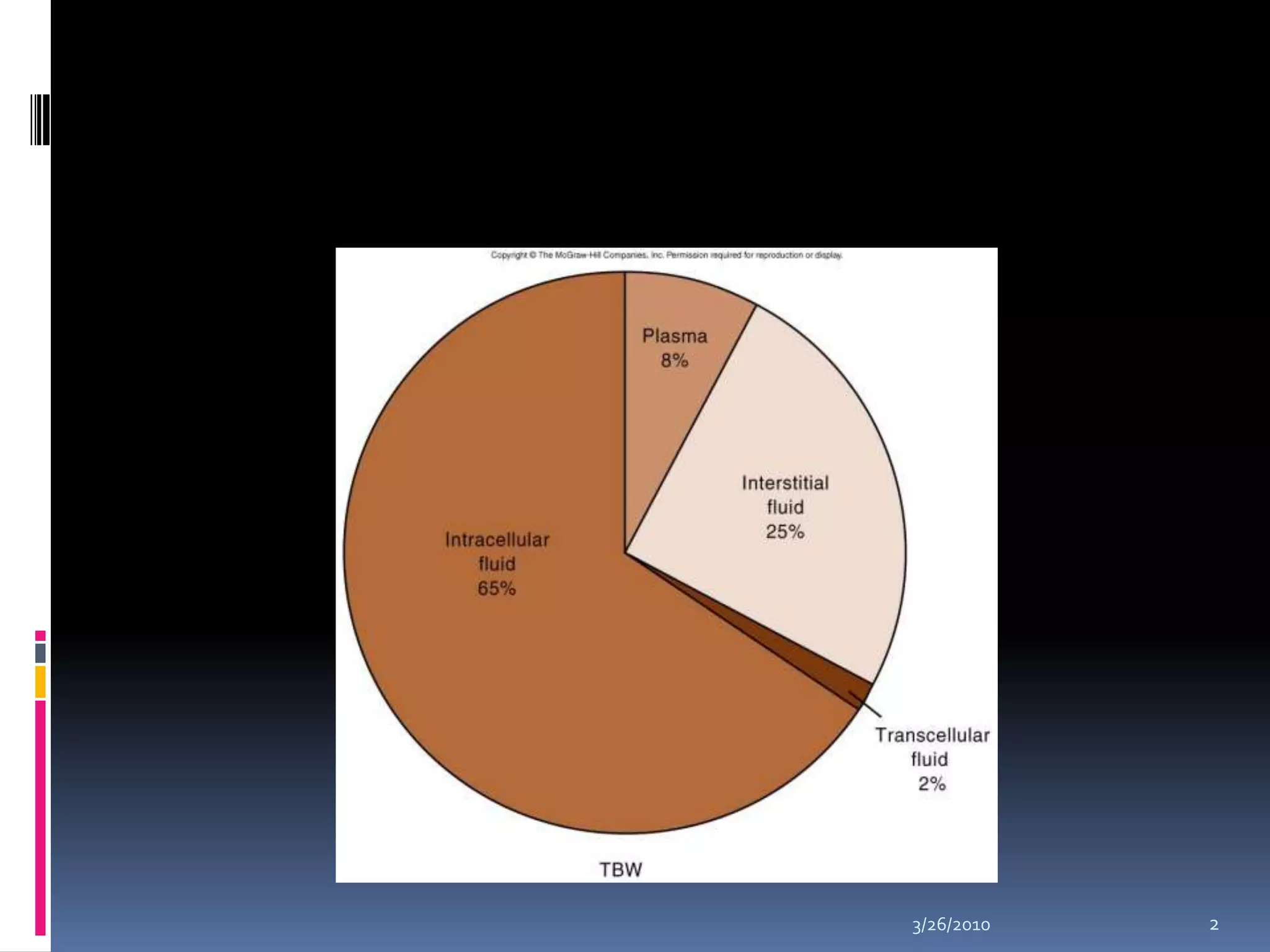
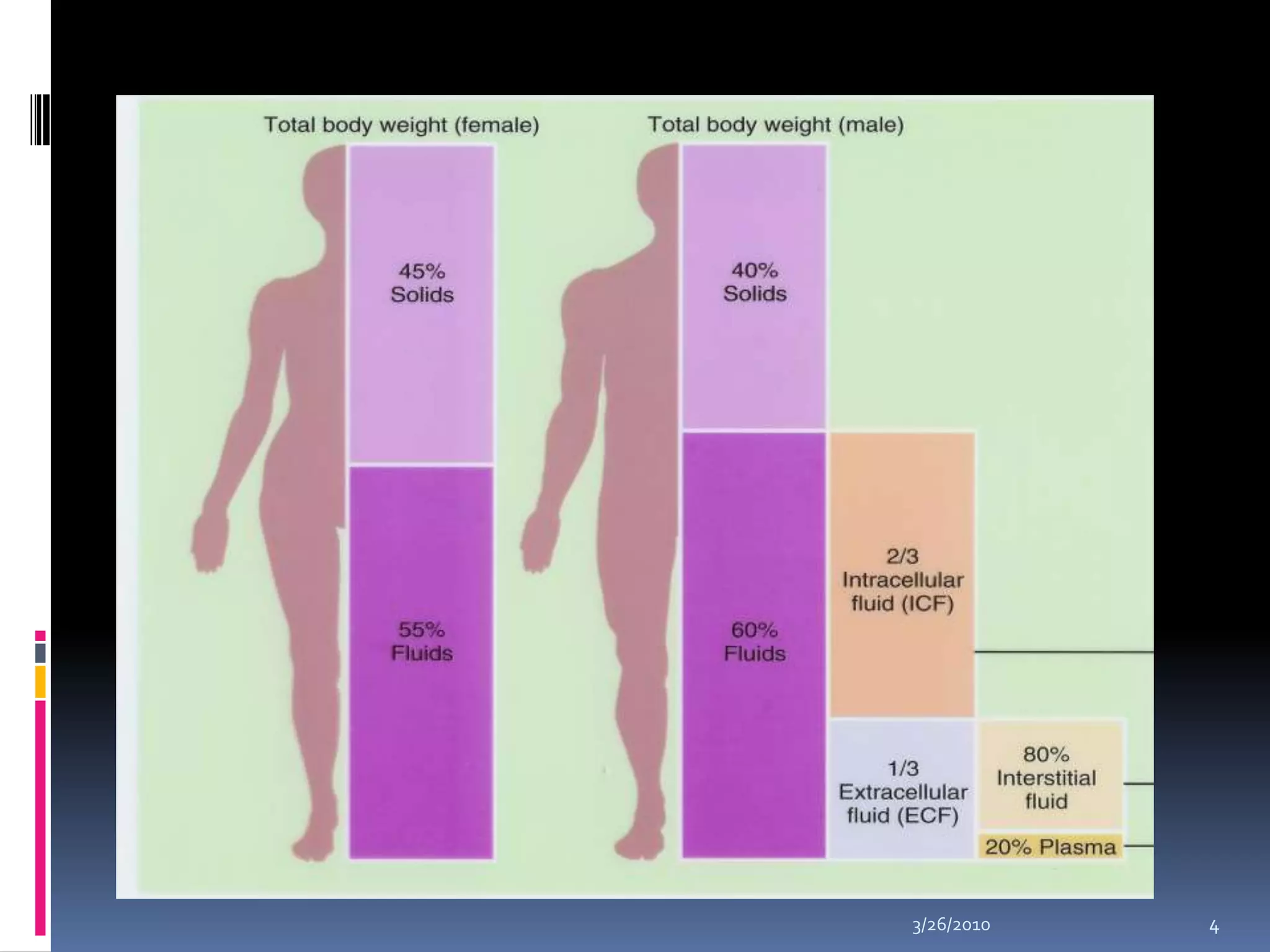
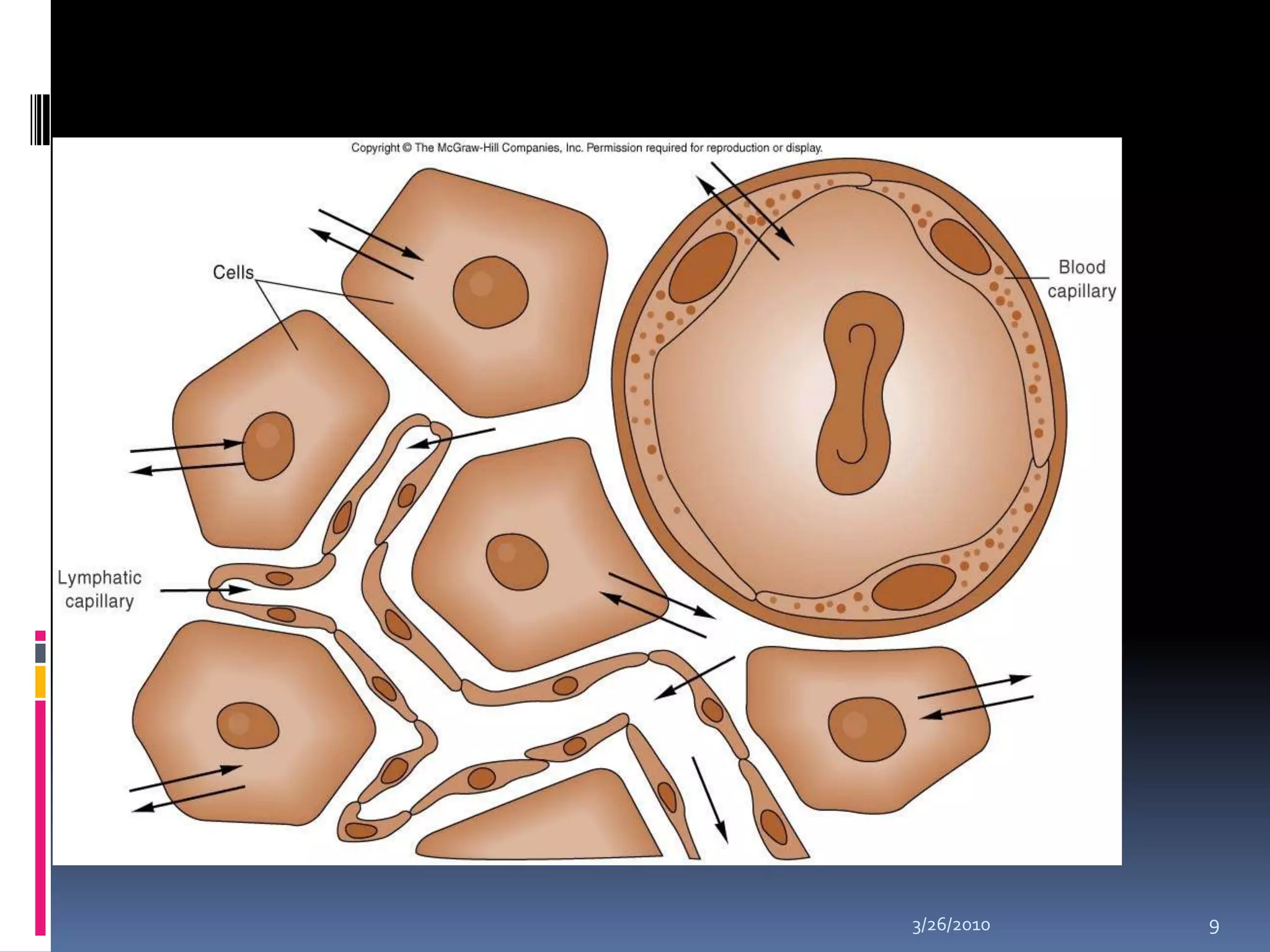
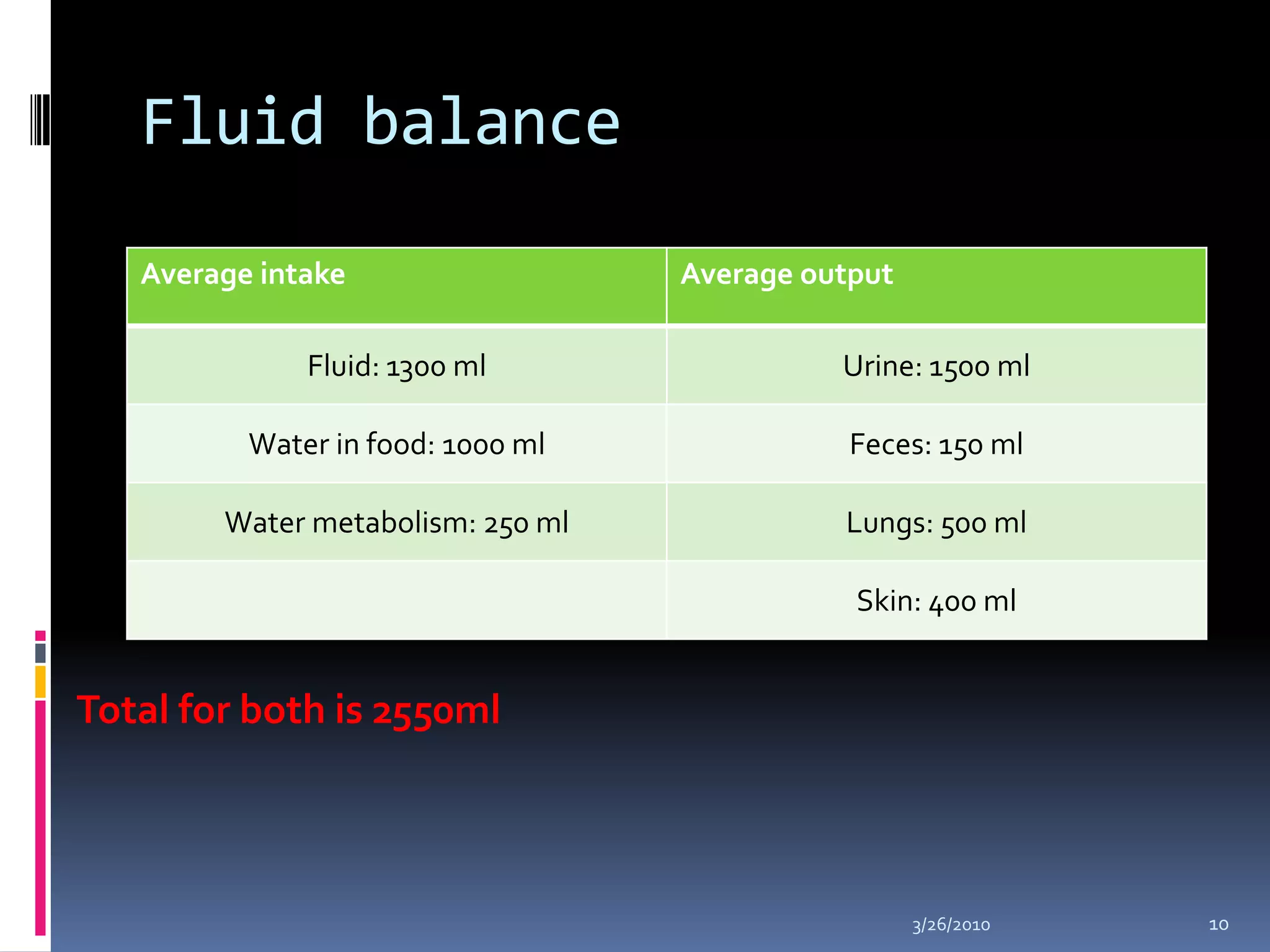
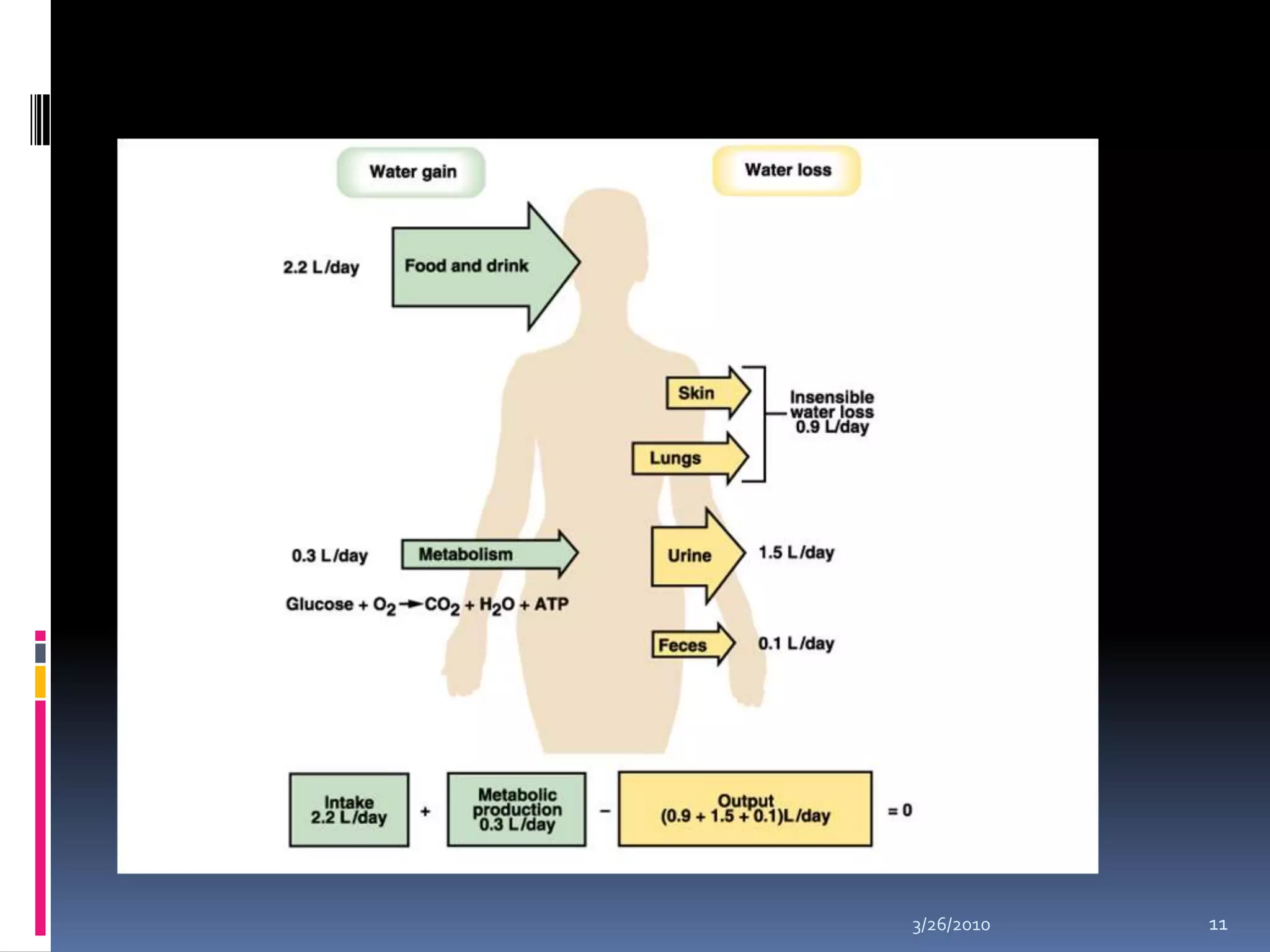
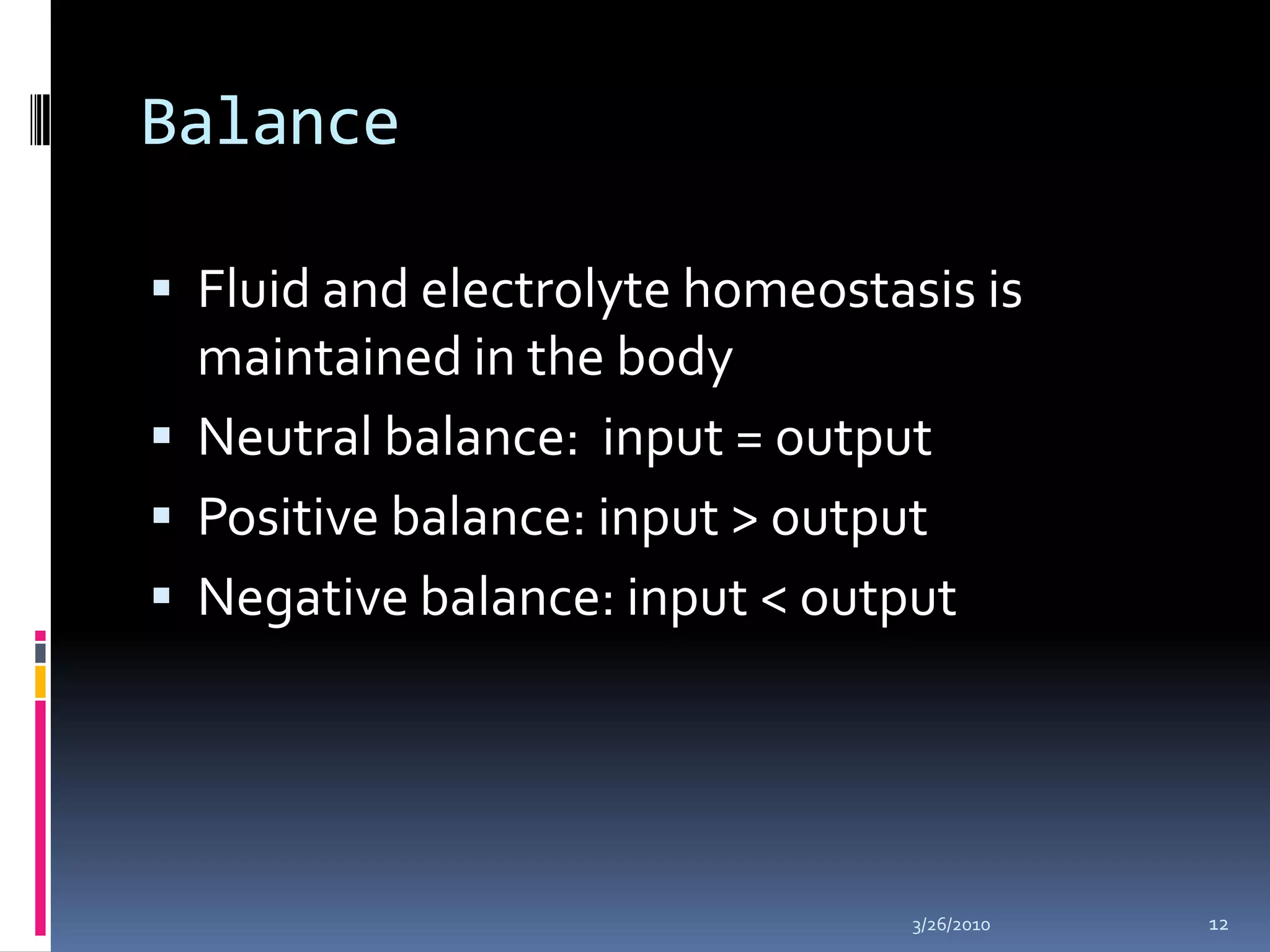
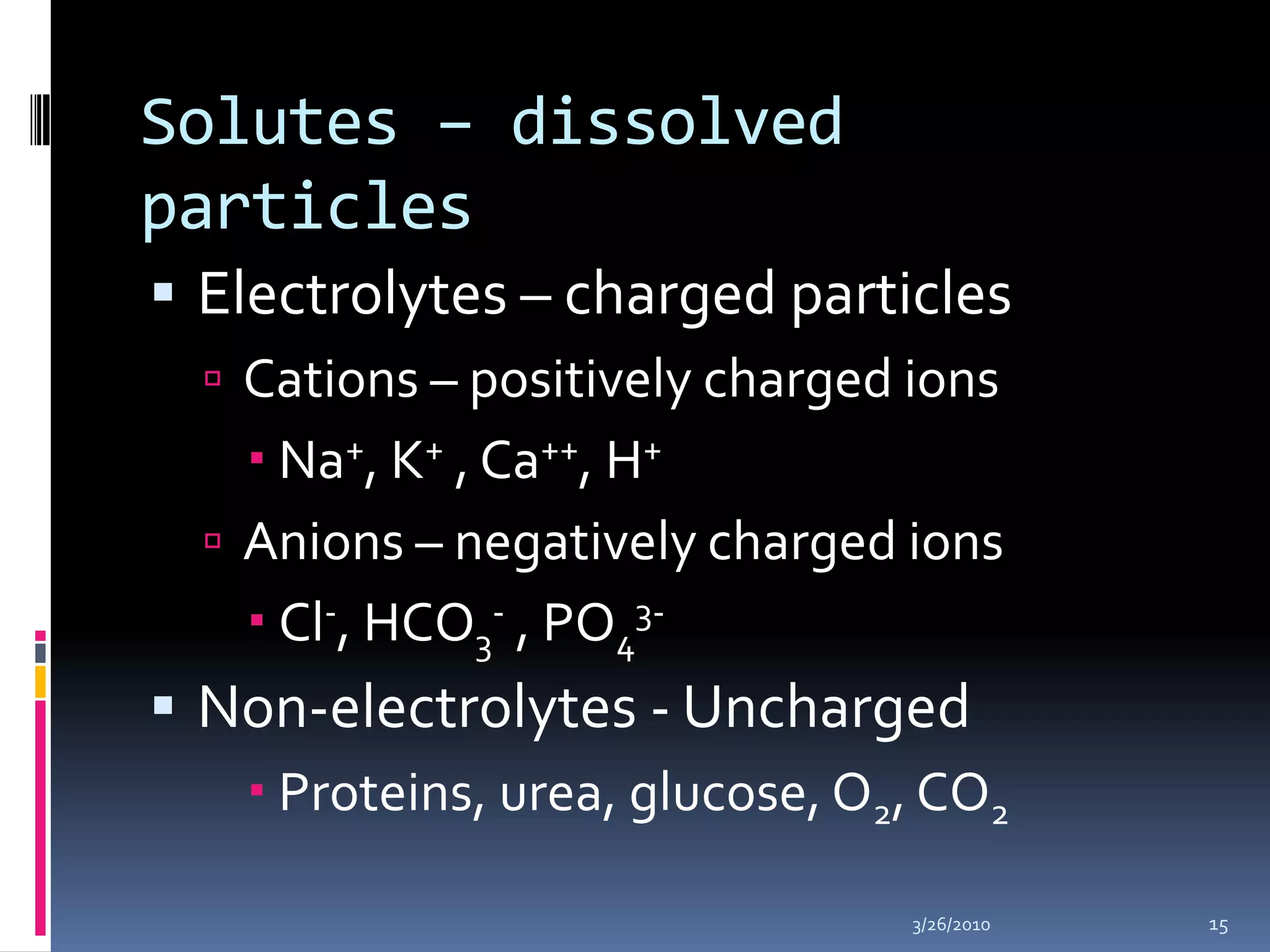
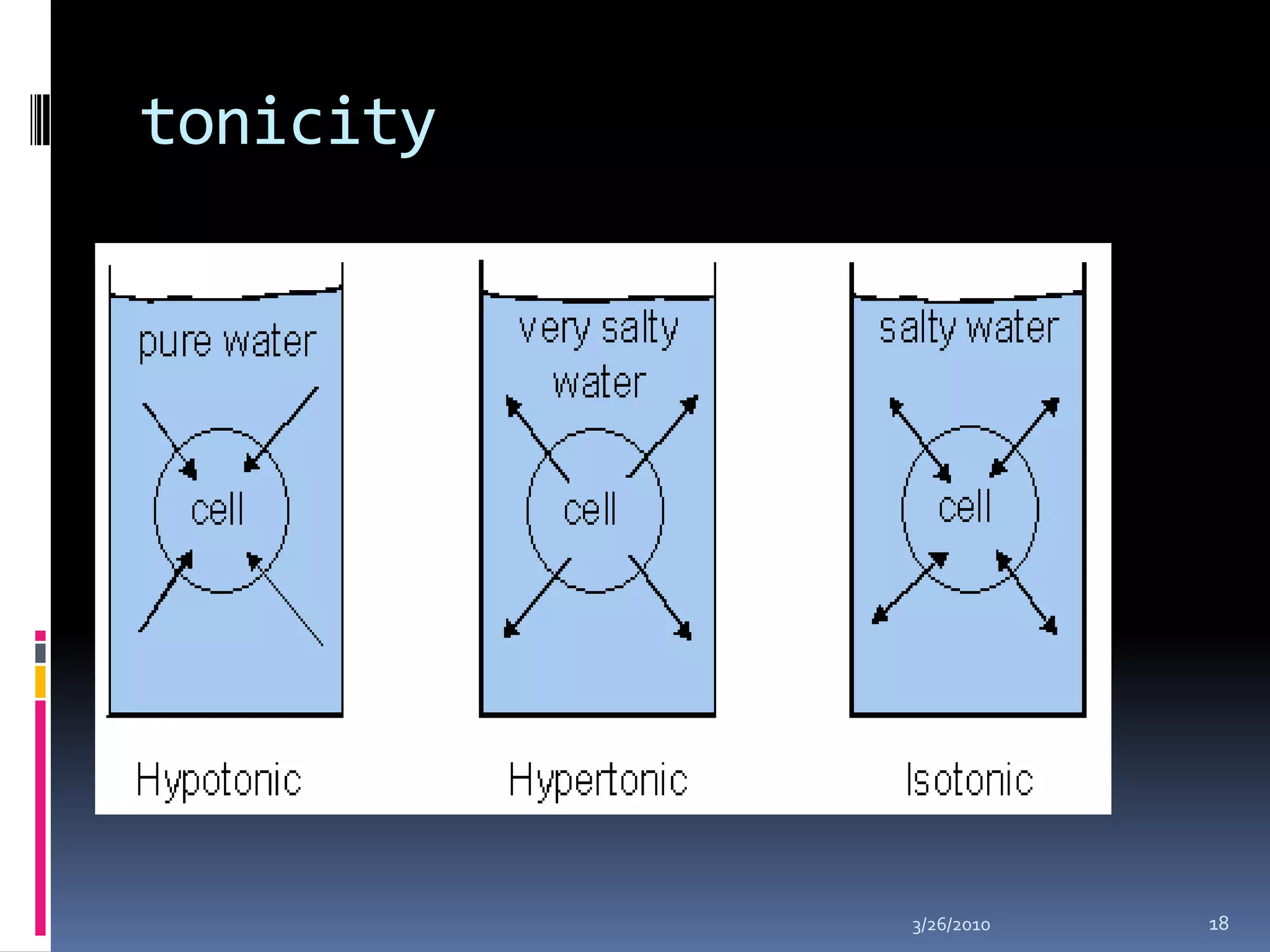
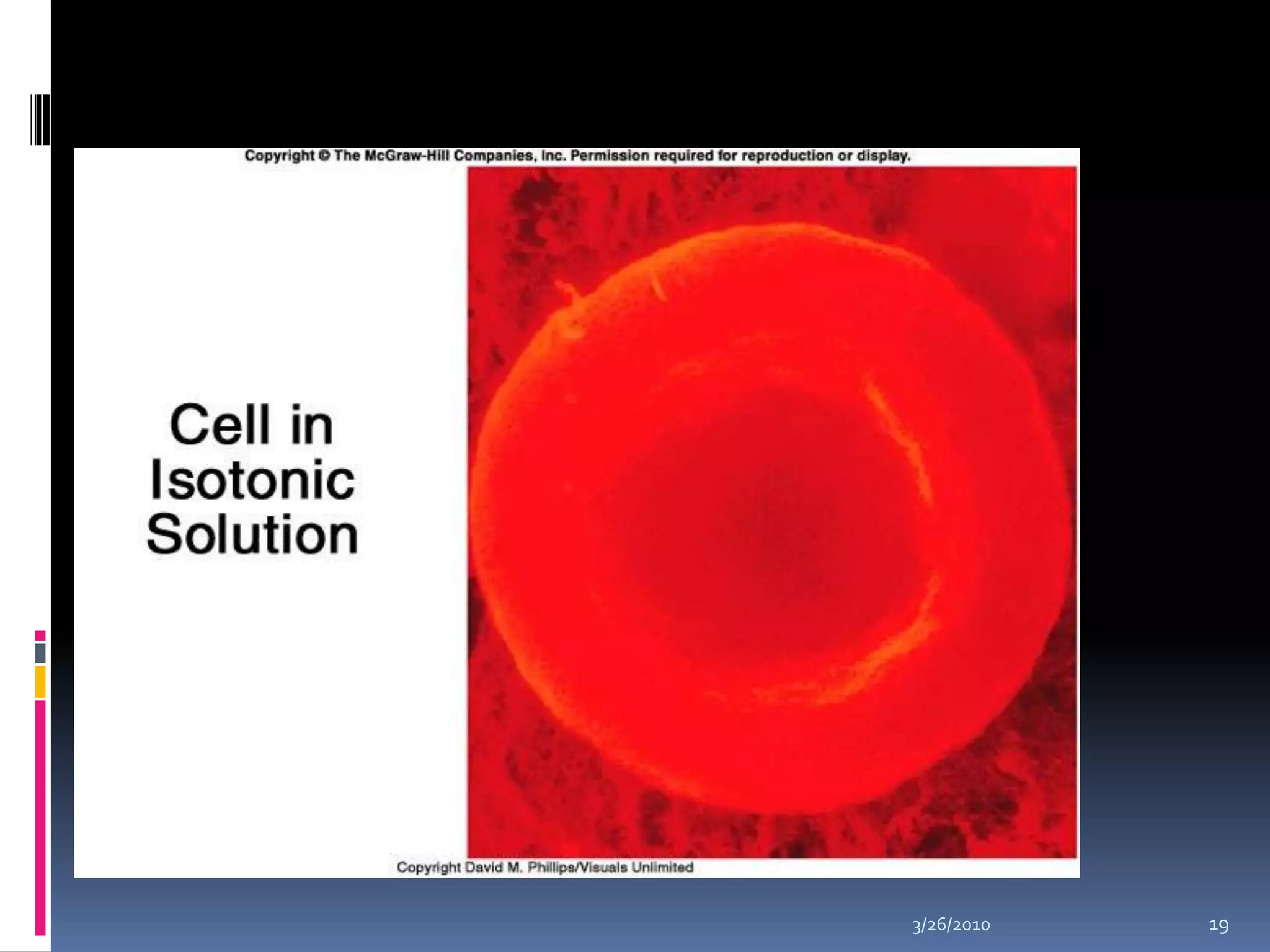
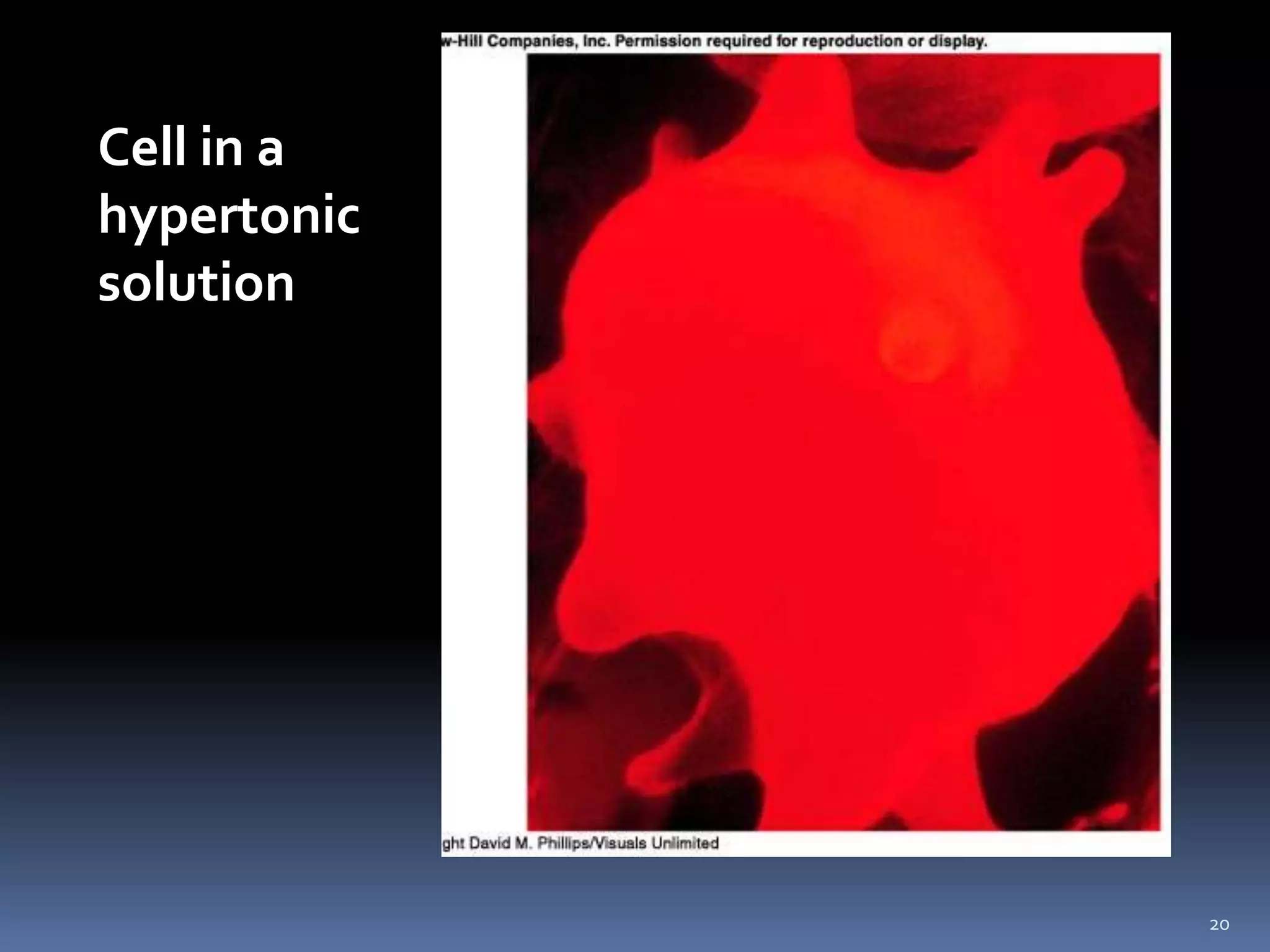
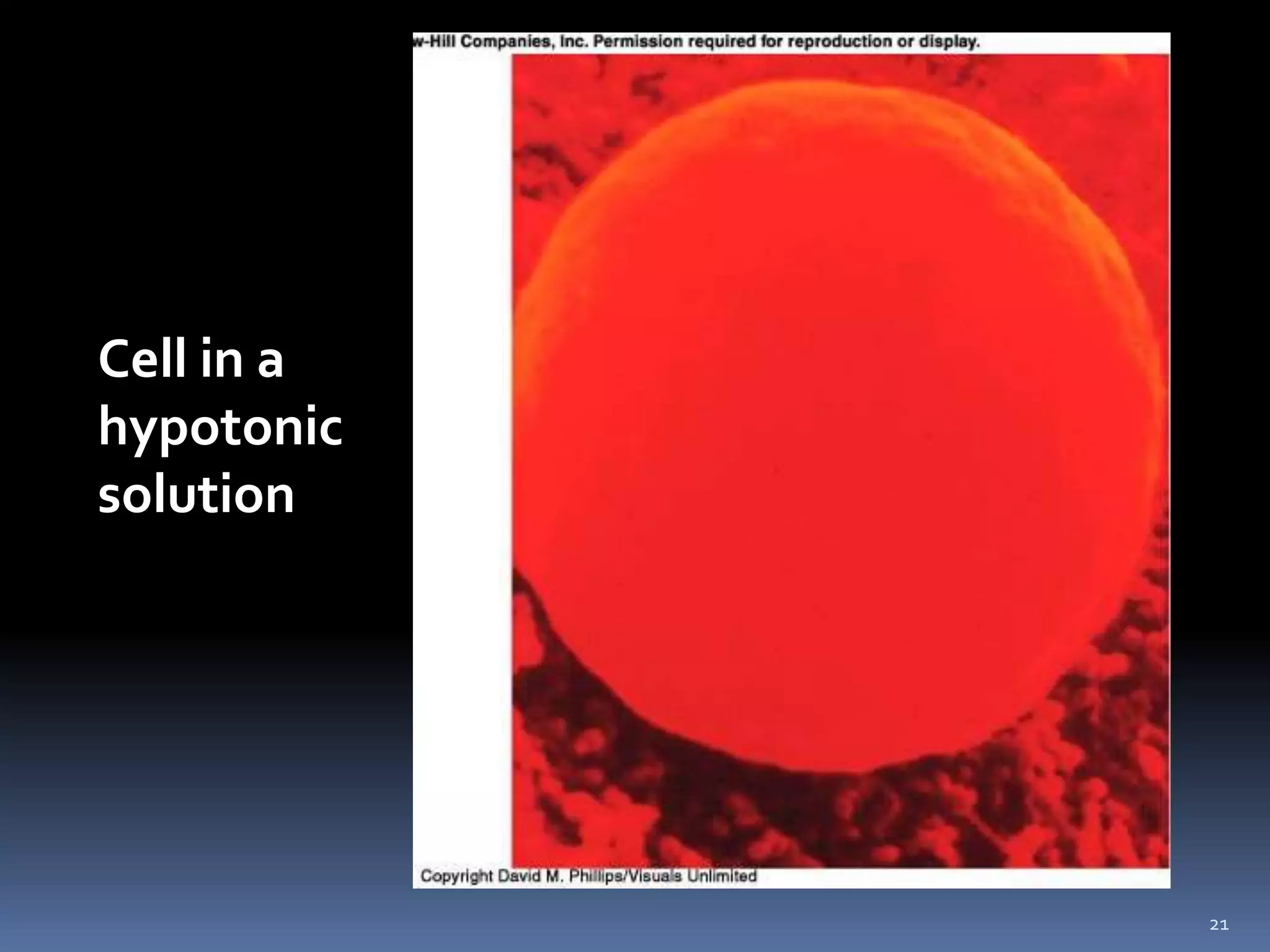
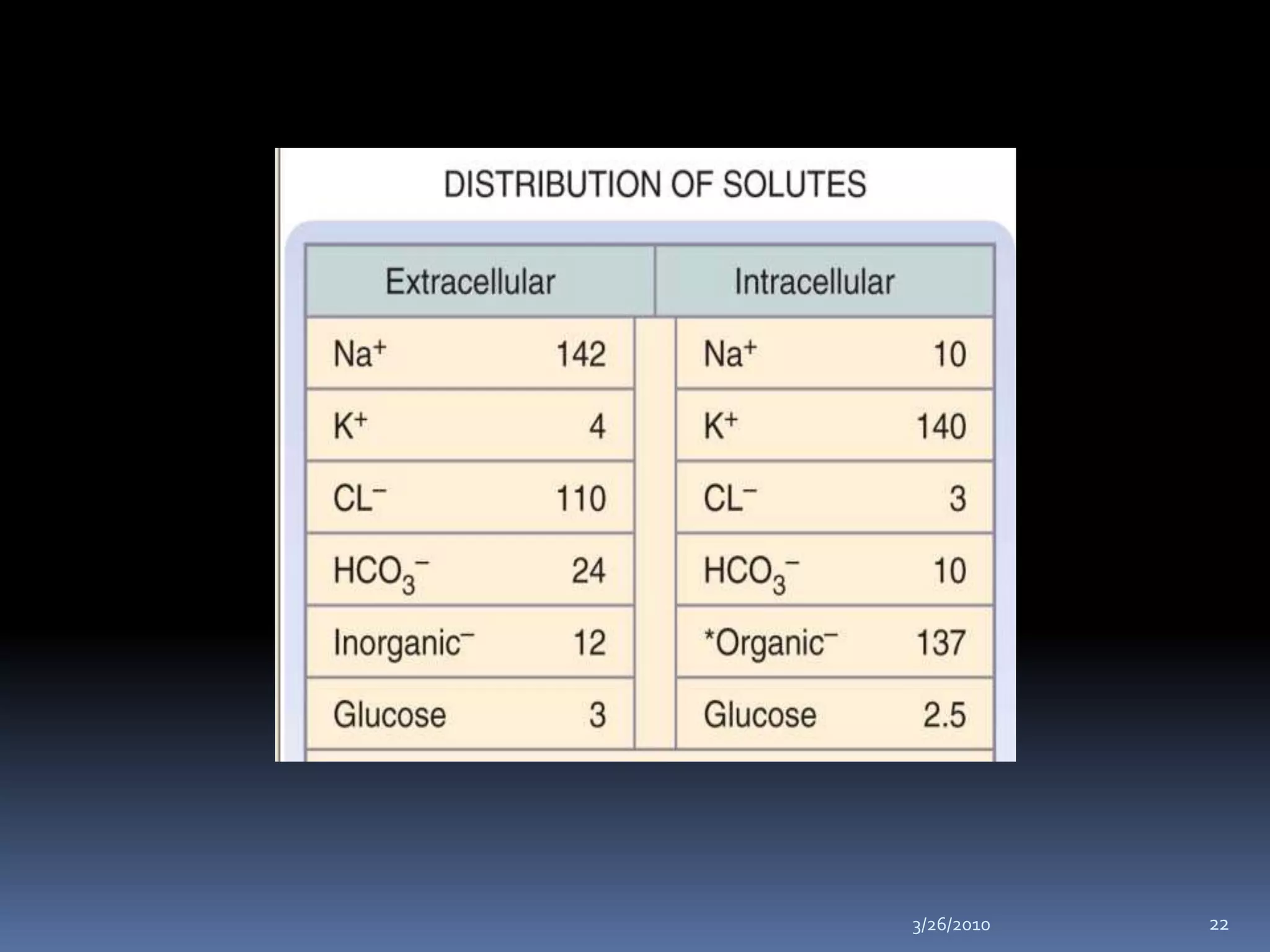
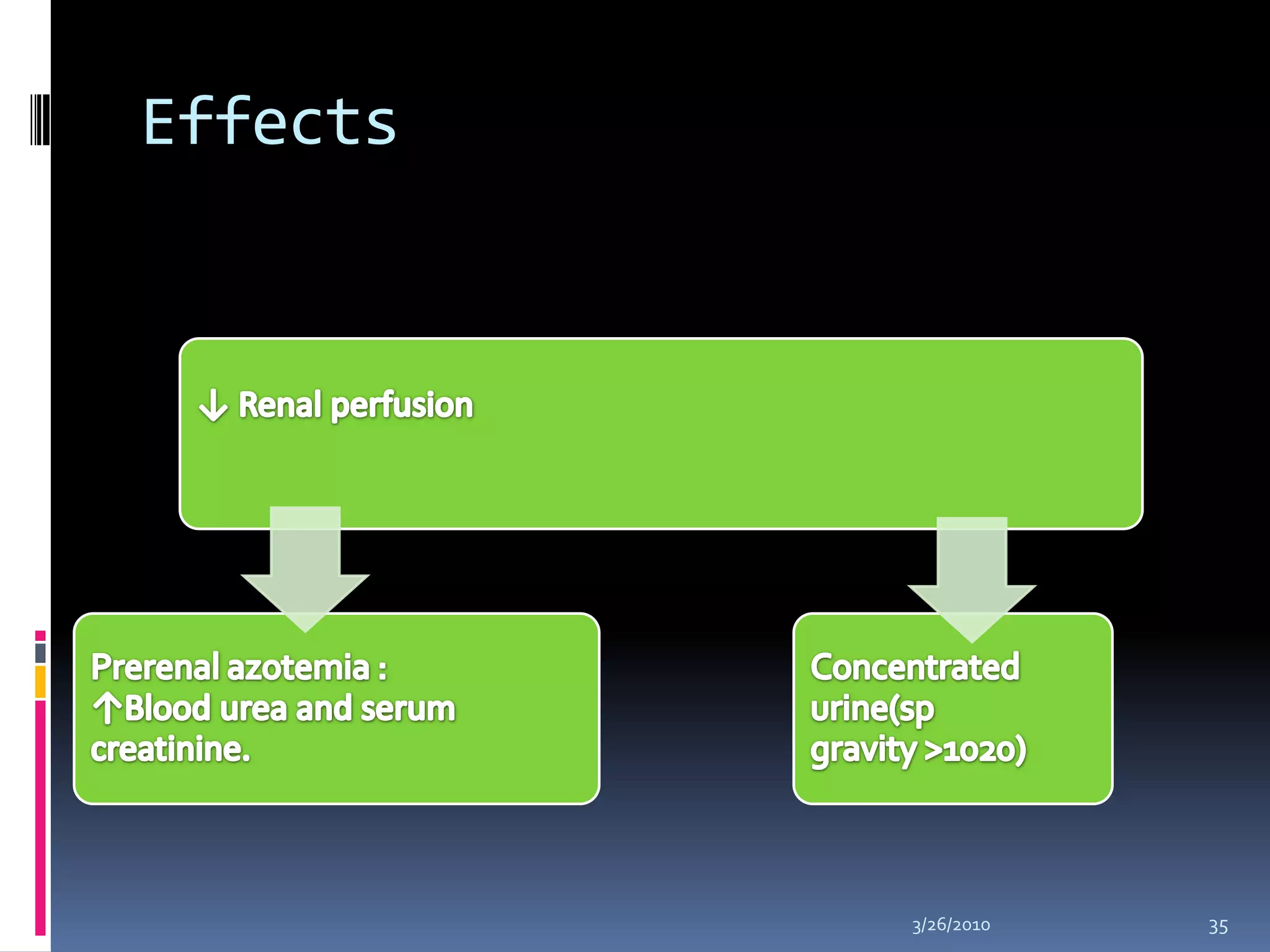
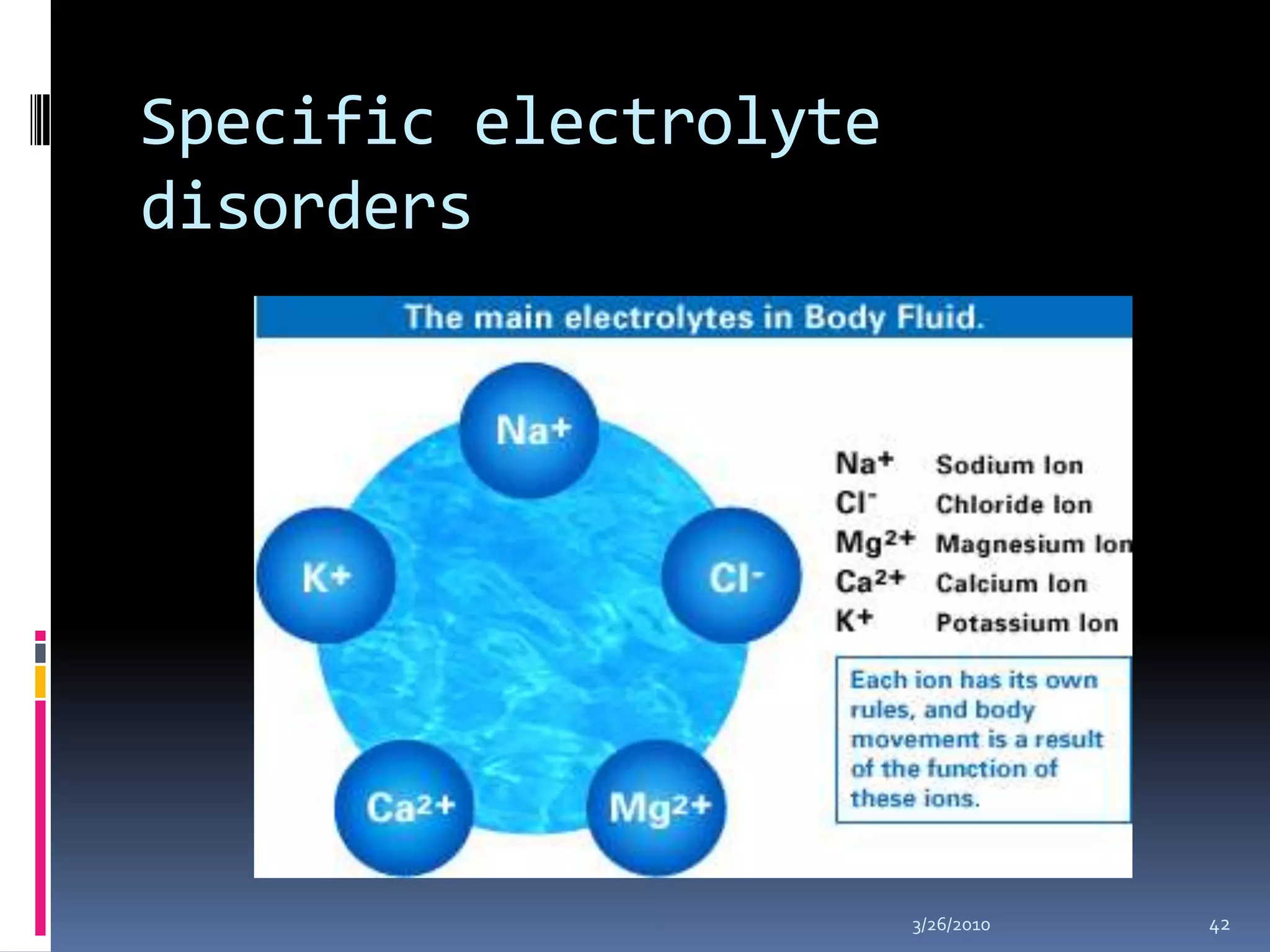
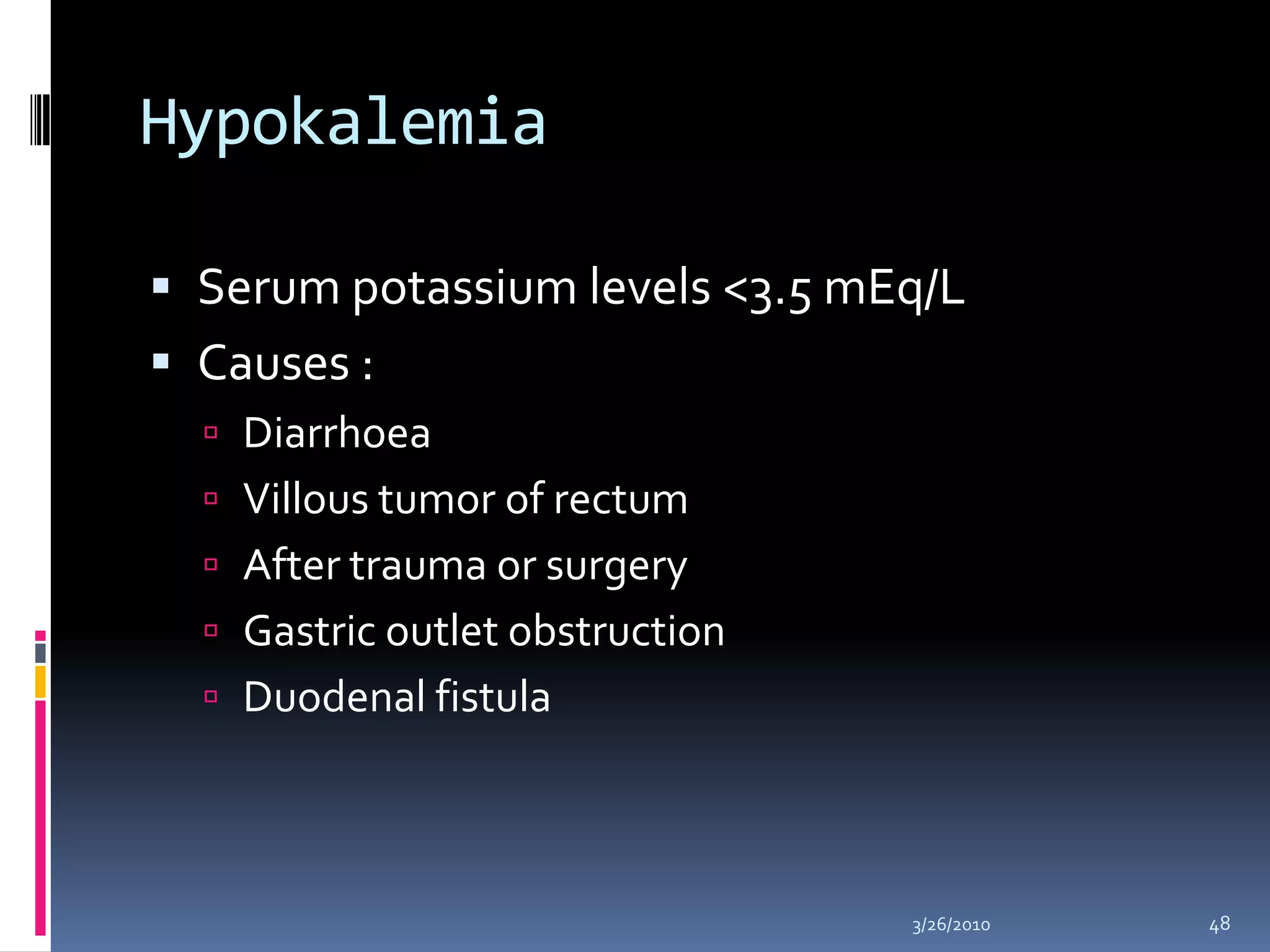
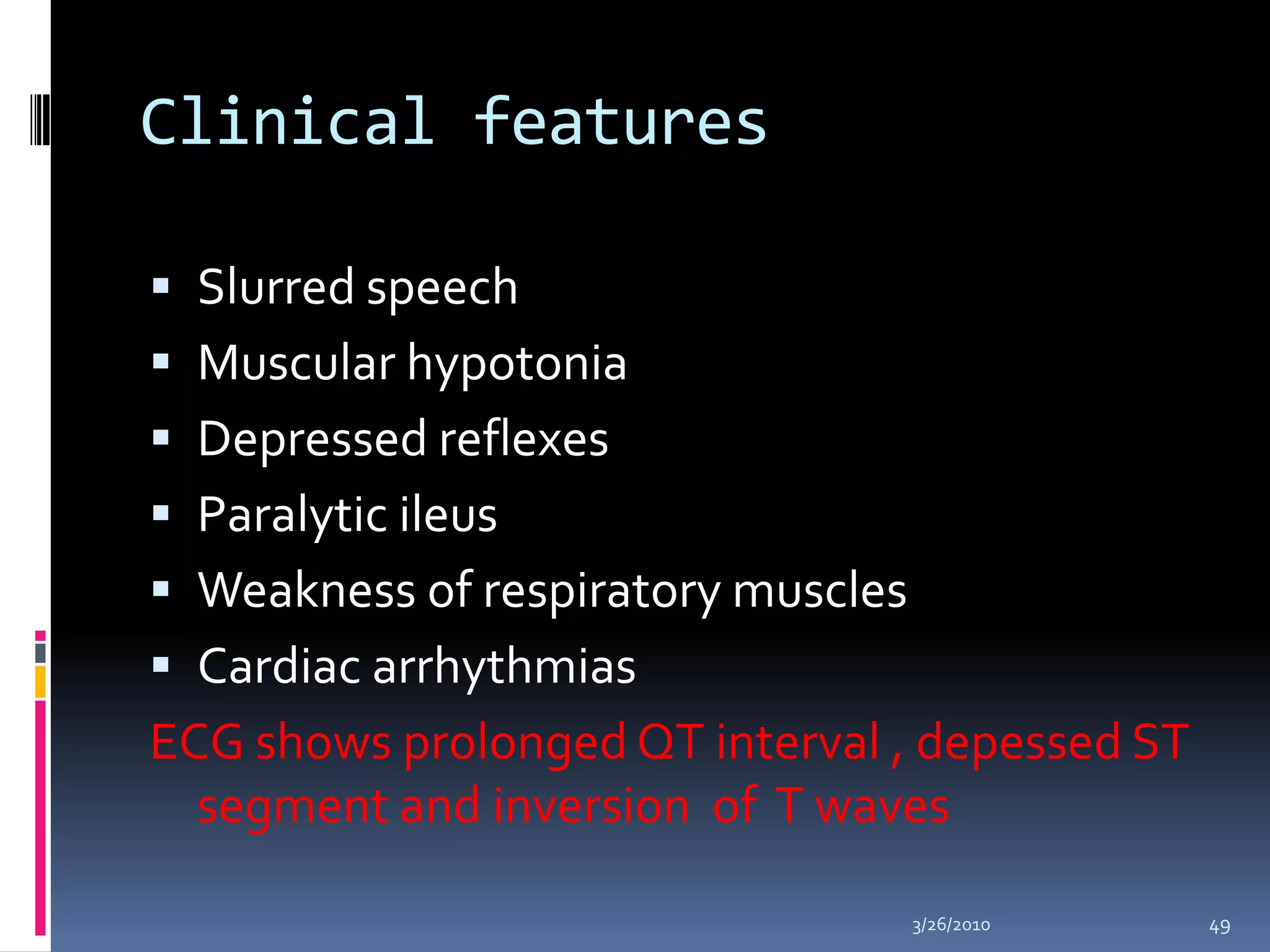
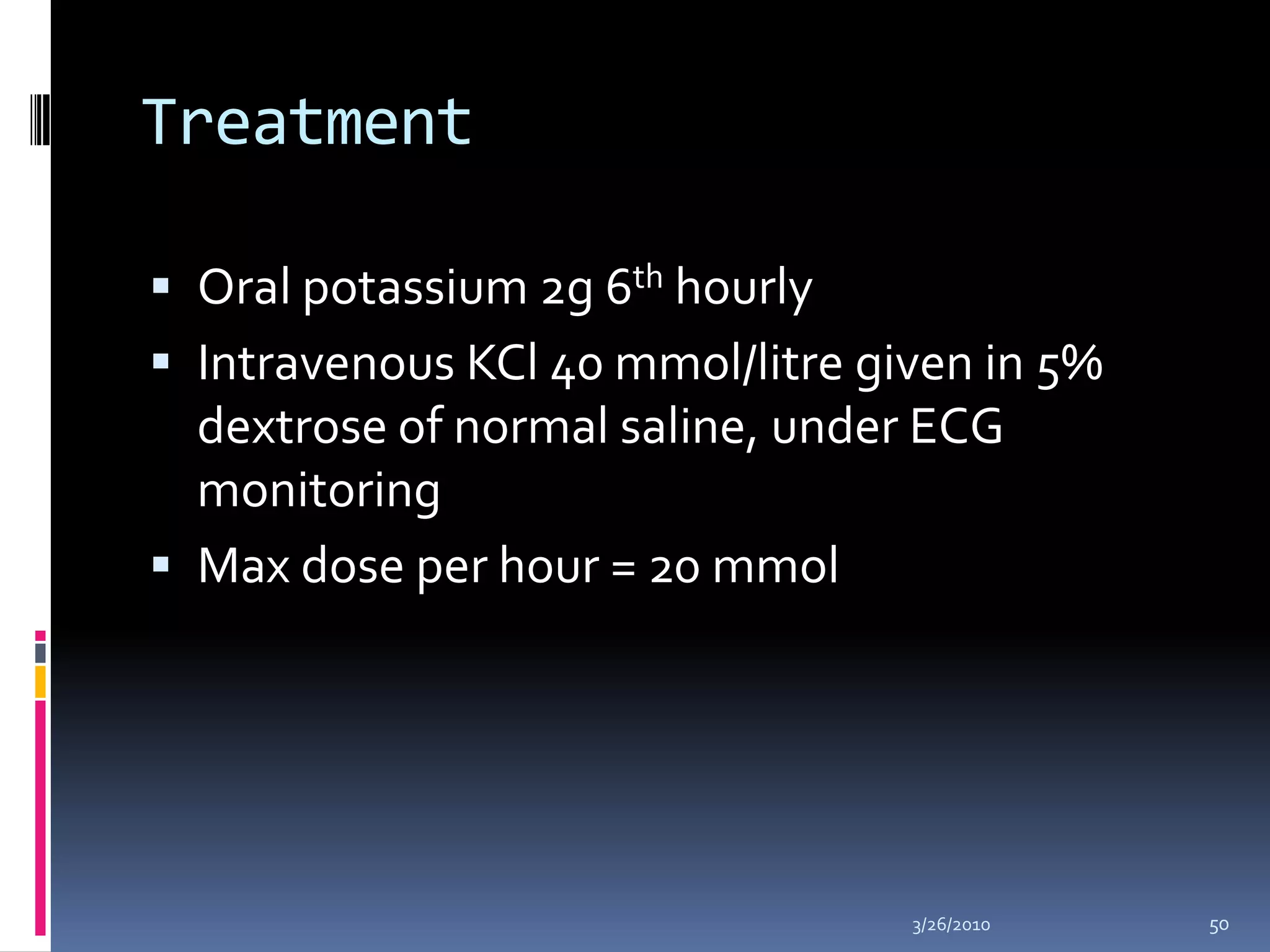
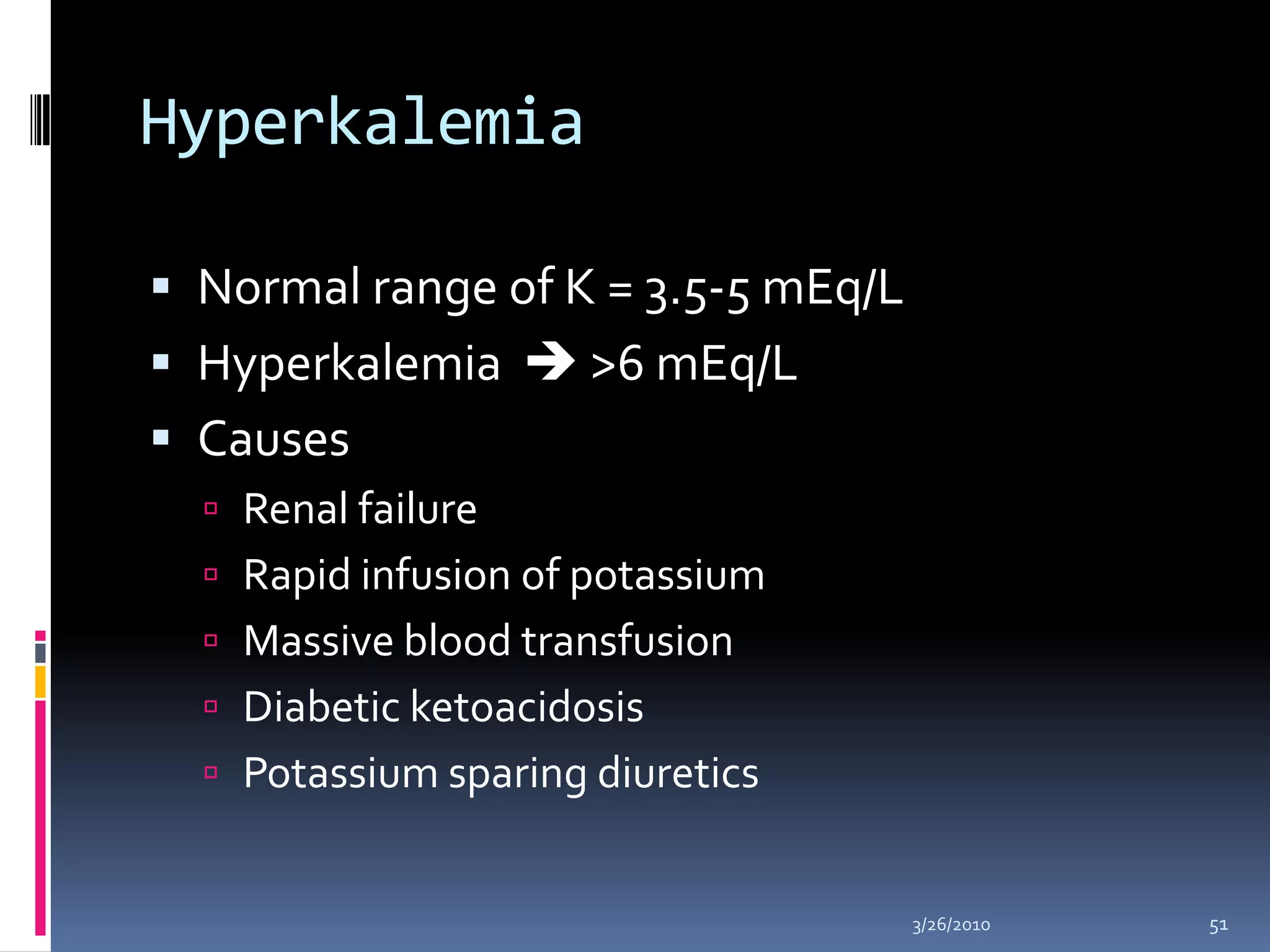
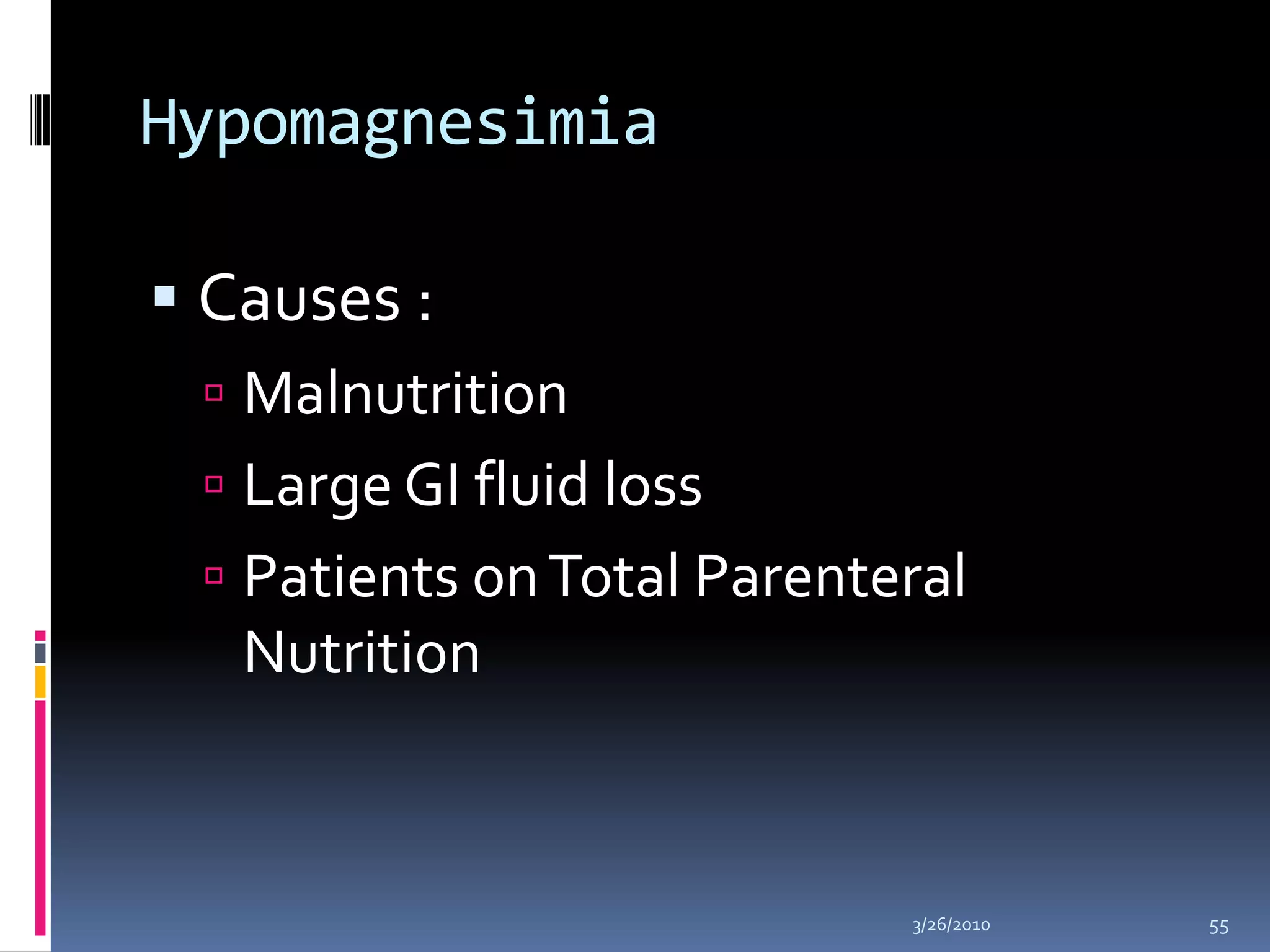
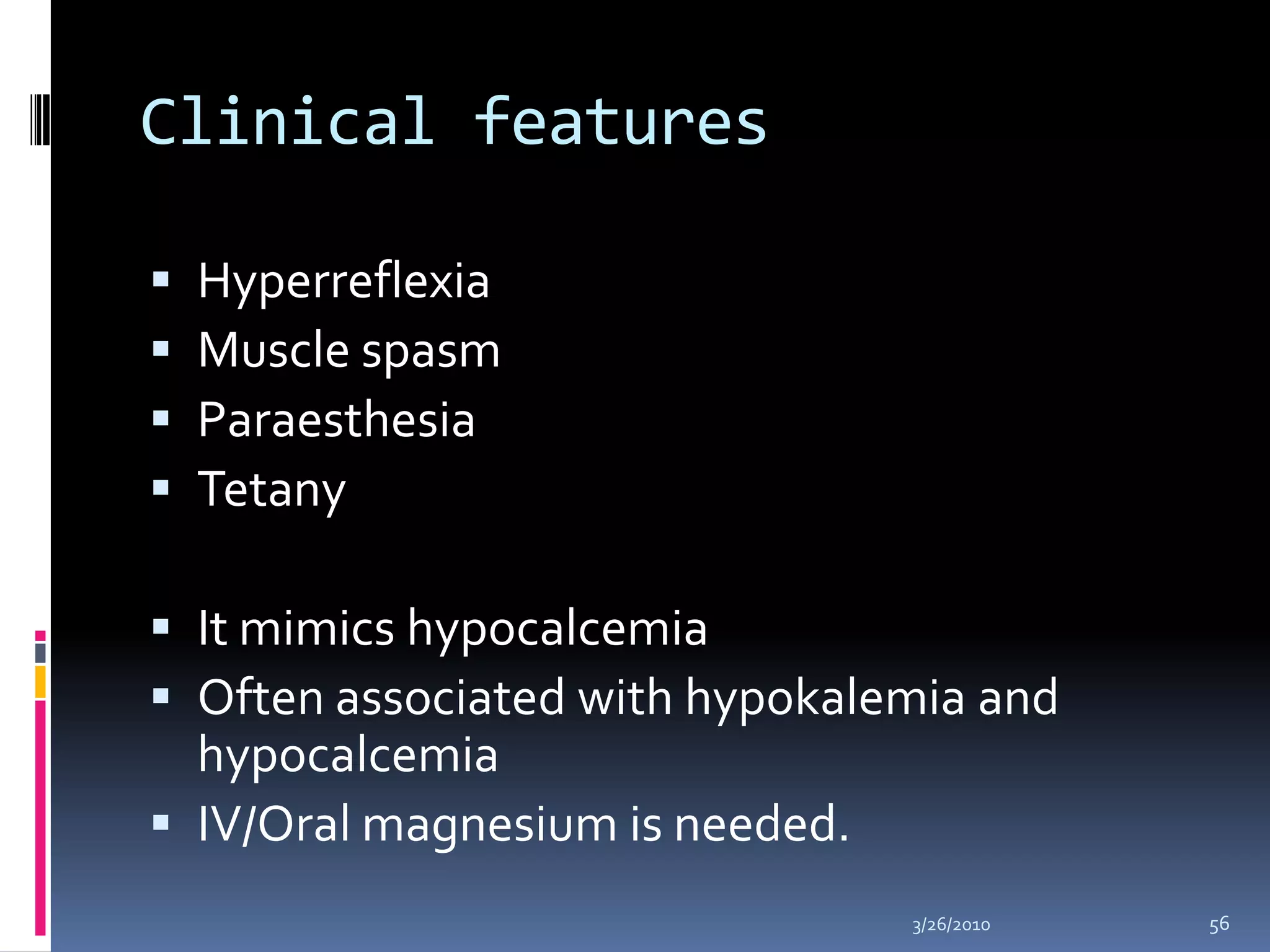
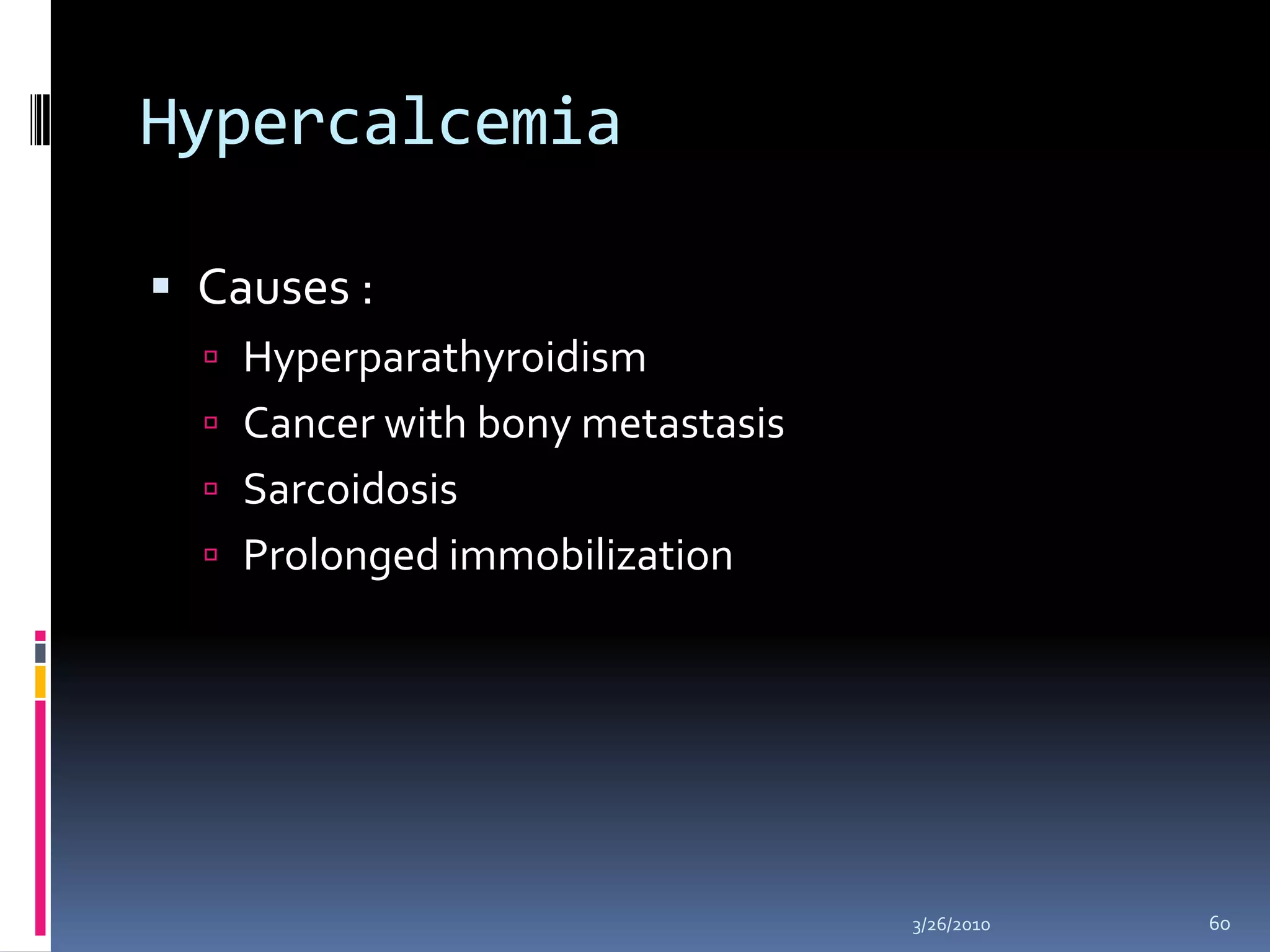
This document discusses fluid compartments and electrolytes. It notes that total body water varies with age, gender, and body fat percentage. Intracellular fluid makes up 60% of body fluid and is rich in potassium and magnesium, while extracellular fluid is 40% and contains more sodium, chloride, and bicarbonate. Fluid compartments are regulated by membranes and pressures. Fluid balance is maintained through neutral, positive, or negative balances. The kidneys, lungs, heart, and hormones like aldosterone and ADH help regulate fluids and electrolytes. Disturbances can cause problems like volume depletion, overload, or specific electrolyte disorders.