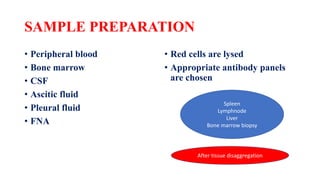
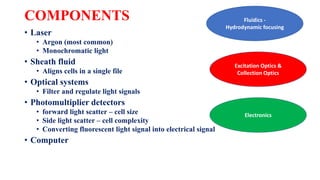
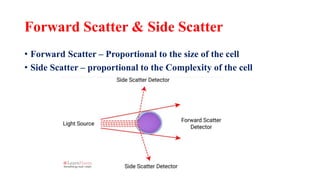
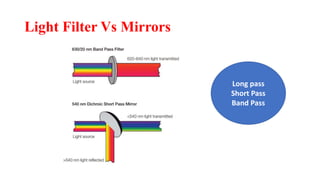
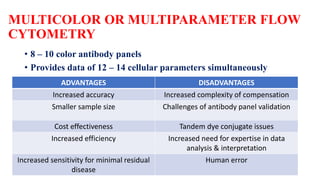
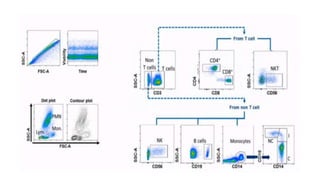
Flow cytometry uses laser-based technology to identify and quantify cell populations in a fluid suspension. Cells flow through the system and are interrogated by detectors that measure their physical properties like size and complexity. It can identify normal vs abnormal cells, differentiate cell types, and quantify tumor infiltration. Key components include lasers, fluidics to align cells, optical systems to filter light signals, and detectors to convert fluorescence into electrical signals for analysis. It allows multiparameter analysis using antibody panels to detect cell surface and intracellular antigens, aiding diagnosis and monitoring of hematological malignancies.