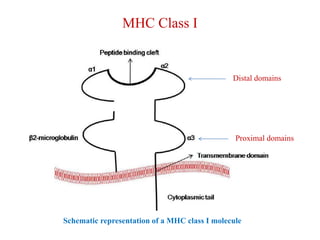
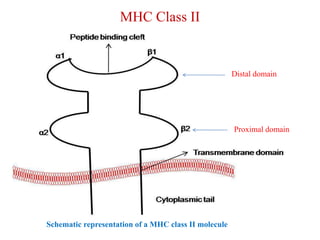
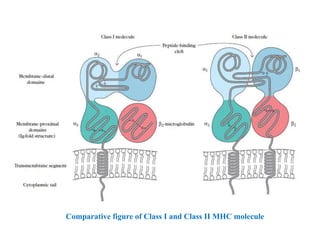
The document summarizes the major histocompatibility complex (MHC), which encodes proteins that play a role in self/non-self discrimination. It describes the structure and function of MHC class I and class II molecules. MHC class I presents endogenous antigens to CD8+ T cells, consisting of an alpha chain, beta-2 microglobulin chain, and a short peptide. MHC class II presents exogenous antigens to CD4+ T cells, consisting of alpha and beta chains that bind longer peptides. Both are membrane-bound glycoproteins that present antigen to T cells via peptide-binding clefts.