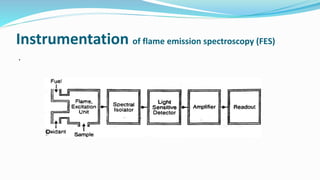
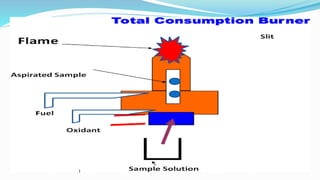
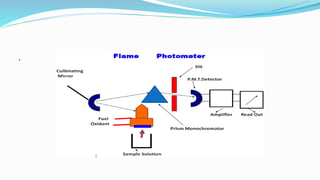
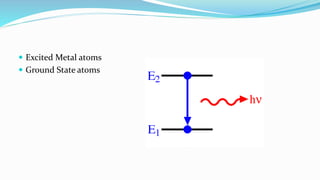
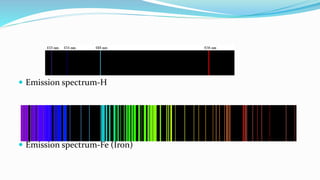
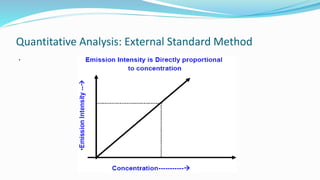
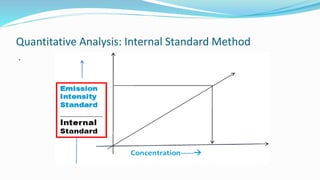
Flame spectrophotometry is a technique that measures the characteristic light emitted by metallic elements when energized, primarily in clinical laboratory settings. The process includes vaporization, atomization, and excitation stages, enabling qualitative and quantitative analysis of metals like sodium and potassium. However, the method has limitations, including a small number of excited atoms, difficulties in controlling flame temperature, and interference from other elements.