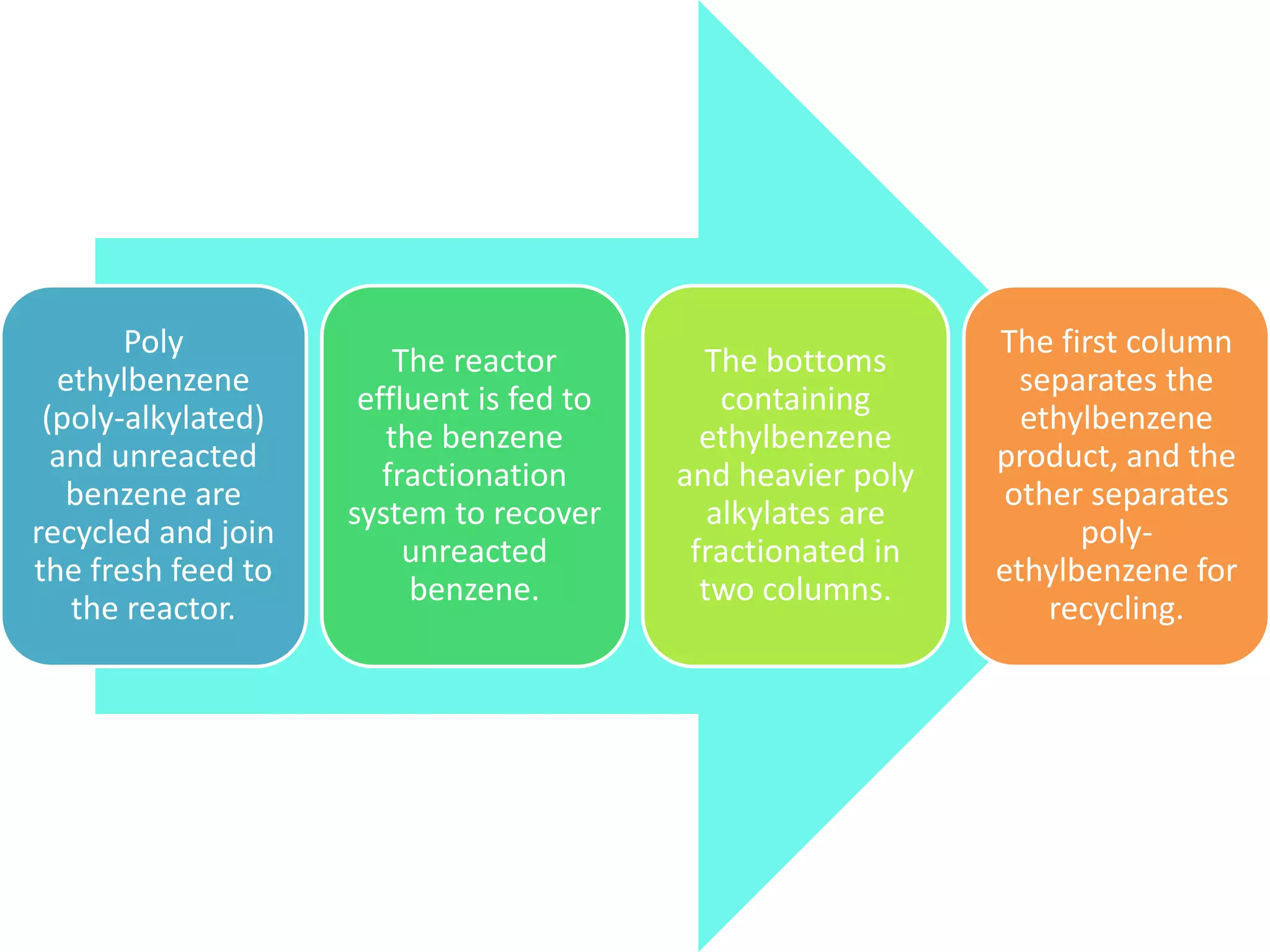
Ethylbenzene was first produced commercially in the 1930s in Germany and the US. It is produced by alkylating benzene with ethylene, such as using the Badger process. Ethylbenzene is over 99% used to produce styrene monomer, which is then used to make many commercial polymers and copolymers. Other minor uses include as a paint solvent or intermediate to produce other chemicals.







![Description Colorless liquid with an aromatic odor (Coty et al., 1987)
Boiling-point 136.2 °C (Lide & Milne, 1996)
Melting-point –94.9 °C (Lide & Milne, 1996)
Density 0.8670 g/cm 3 at 20 °C (Lide & Milne, 1996)
Spectroscopy
data
Infrared, ultraviolet [97], nuclear magnetic resonance and mass spectral
data have been reported (Lide & Milne, 1996)
Solubility Slightly soluble in water (152 mg/L at 20 °C) (ECETOC, 1986) and
chloroform; miscible with diethyl ether and ethanol (Lide & Milne,
1996)
Volatility Vapor pressure, 1.28 kilo Pascal at 25 °C (Lide & Milne, 1996); relative
vapor density (air = 1), 3.7 (Verschueren, 1996); flash-point (closed-
cup), 15 °C (Coty et al., 1987)
Octanol/water
partition
coefficient (P)
log P, 3.15 (Verschueren, 1996)
Conversion
factor
mg/m 3 = 4.34 × ppm](https://image.slidesharecdn.com/prinatable1-170331025503/75/Ethyl-Benzene-8-2048.jpg)