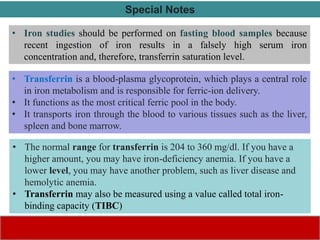
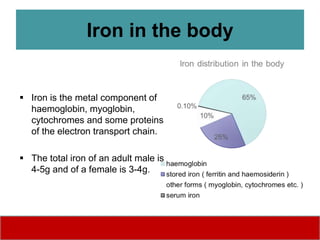
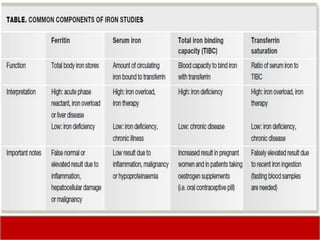
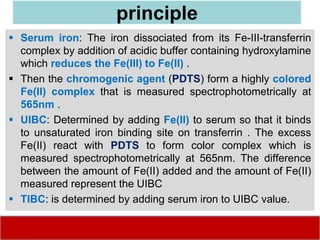
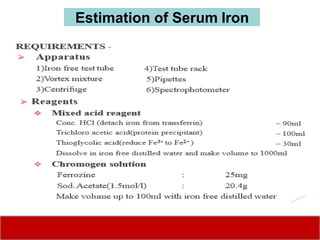
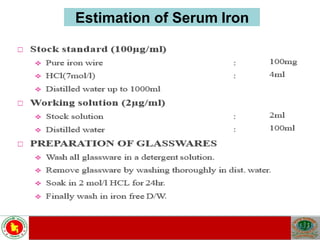
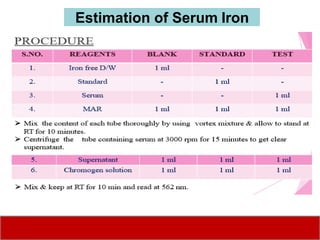
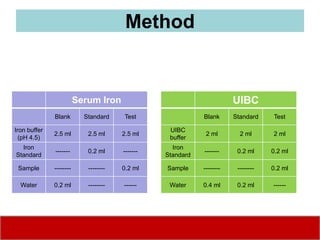
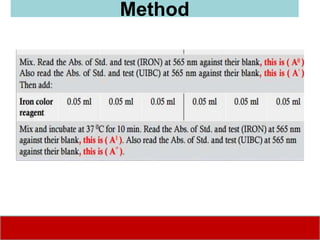
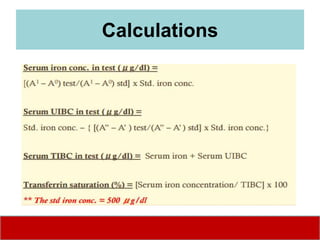
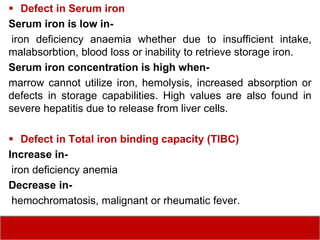
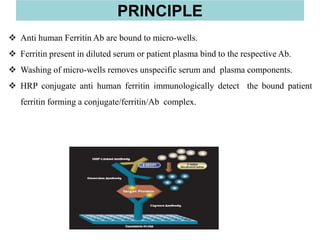
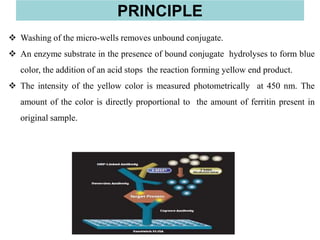
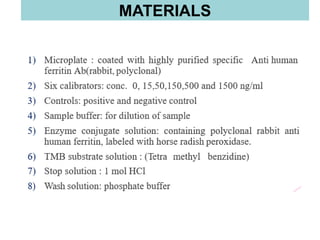
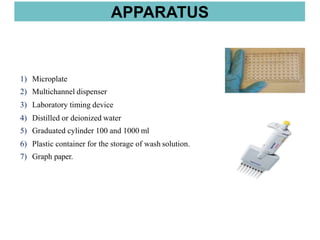
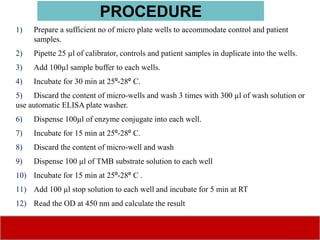
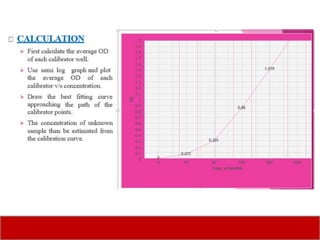
This document discusses laboratory tests for serum iron, total iron binding capacity (TIBC), transferrin, ferritin, vitamin B12 and serum folate. It provides objectives, principles, methodologies, normal ranges and limitations for each test. The tests are useful in diagnosing iron deficiency anemia and vitamin deficiencies. Sample collection and handling factors that could impact results are also outlined.