This document describes the process for estimating haemoglobin levels through the cyanomethaemoglobin (HiCN) method. Key points:
- Haemoglobin transports oxygen and carbon dioxide in red blood cells. The cyanomethaemoglobin method is the standardized way to estimate haemoglobin concentration.
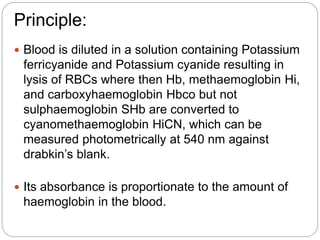
- In this method, blood is diluted with Drabkin's solution containing potassium ferricyanide and potassium cyanide. This lyses red blood cells and converts haemoglobin to stable cyanomethaemoglobin, which can be measured photometrically.
- Absorbance readings of test samples are compared to a standard to calculate haemoglobin concentration using Beer's law. Normal ranges vary by age, sex and