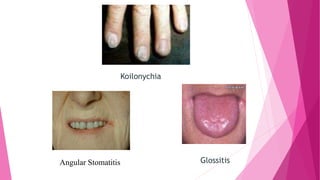
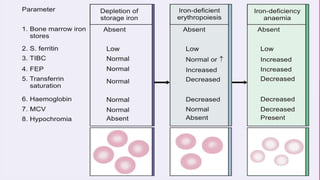
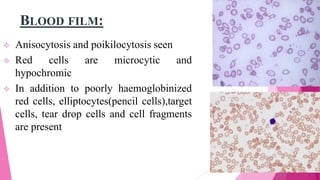
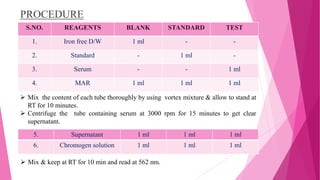
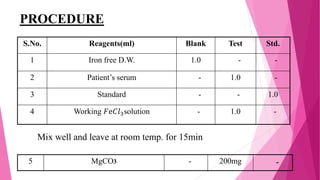
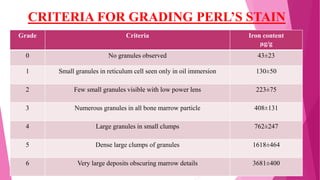
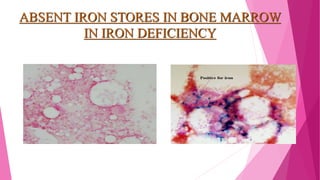
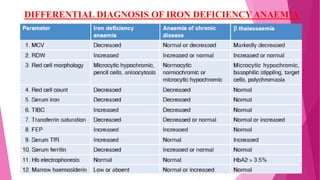
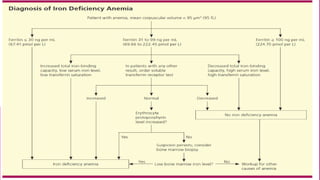
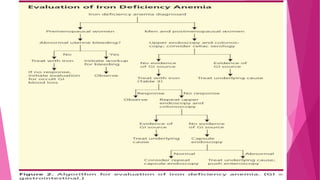
Anaemia is defined by the WHO as a condition where the number of red blood cells or their oxygen-carrying capacity is inadequate for physiological needs. Iron deficiency anaemia (IDA) occurs when the bone marrow lacks sufficient iron, resulting in small, pale erythrocytes, and is prevalent in various demographics in India, particularly among women and children. Laboratory diagnosis involves assessing serum ferritin and transferrin levels, with specific clinical features including fatigue, koilonychia, and pica.