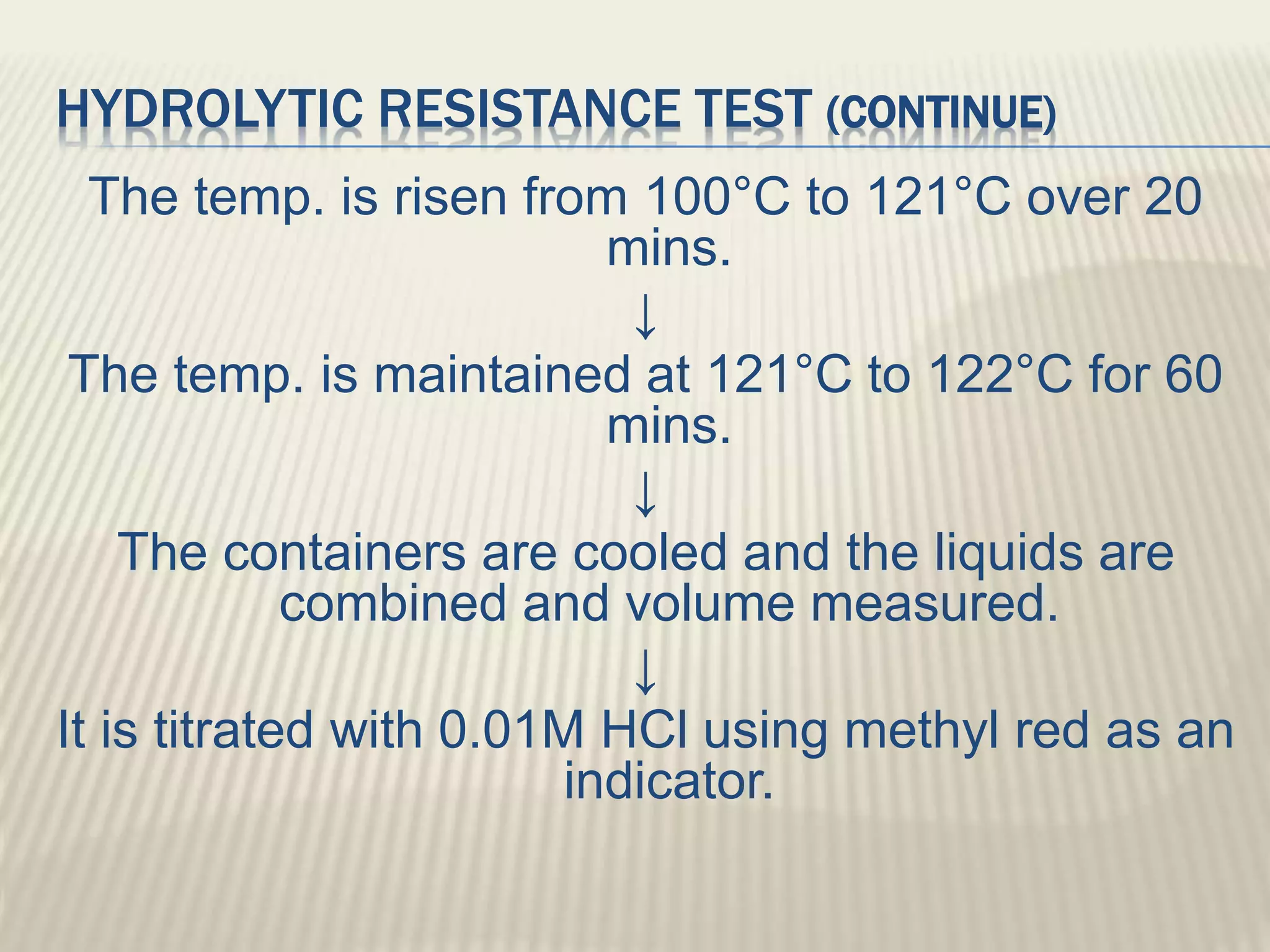
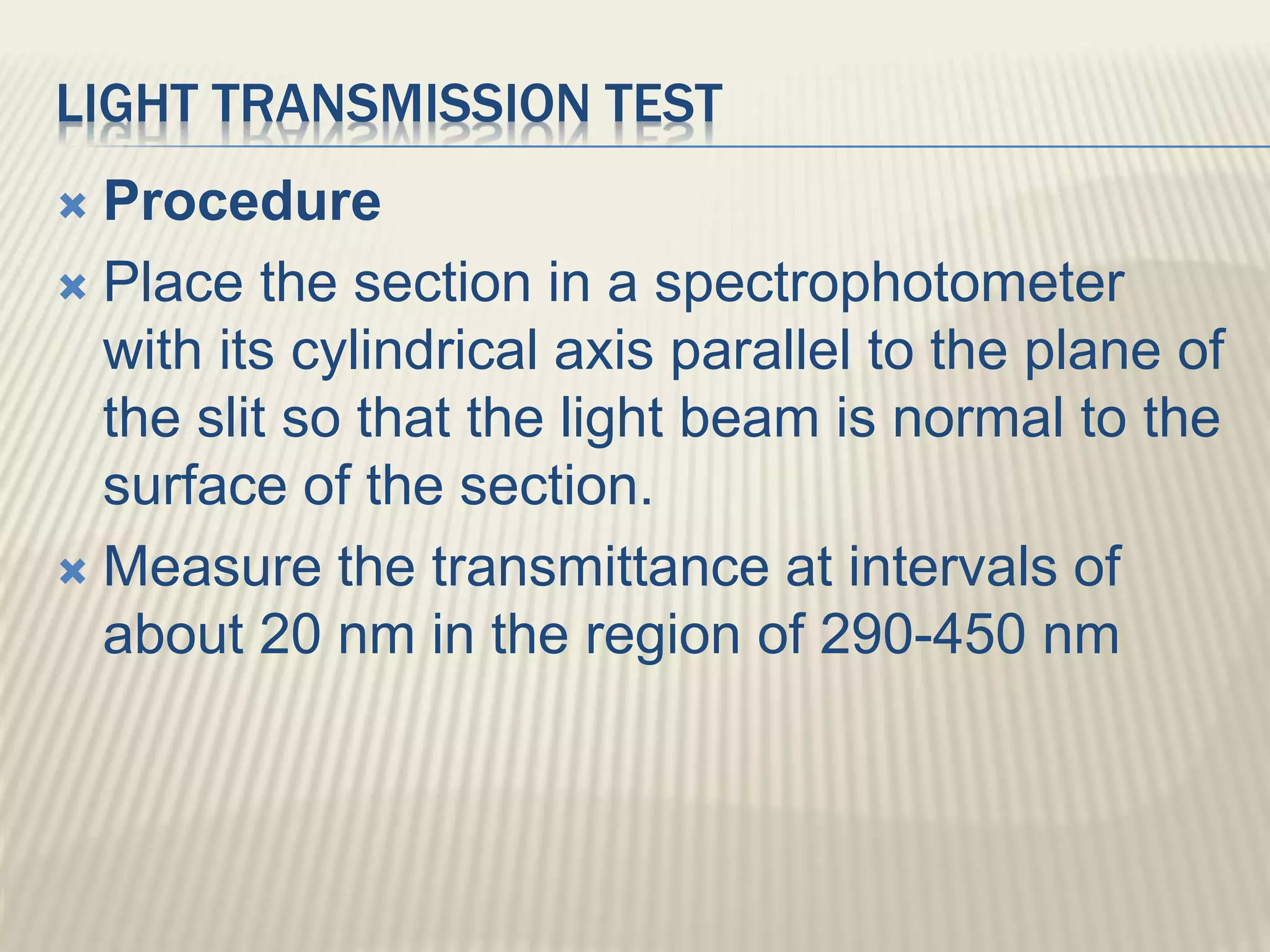
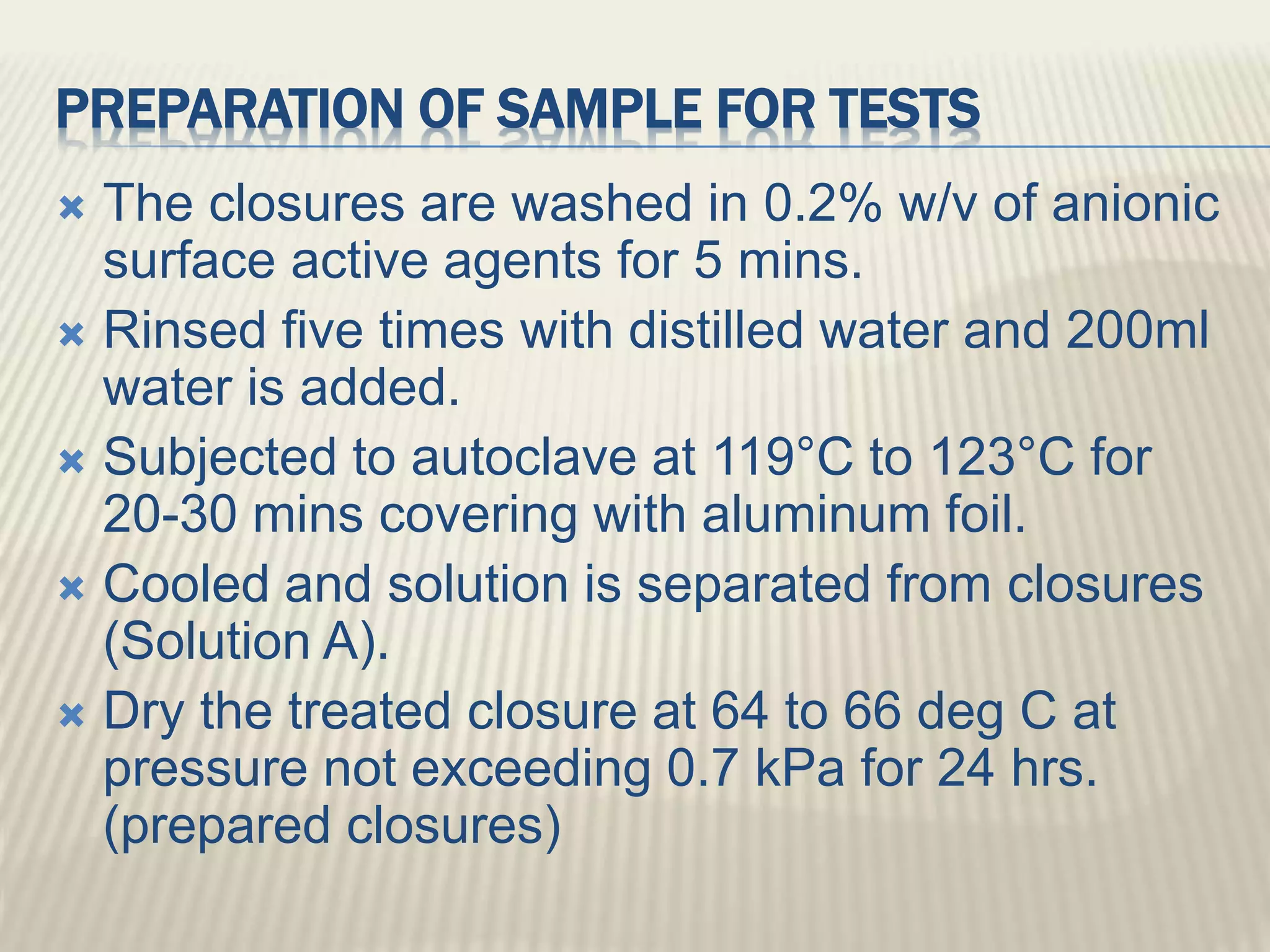
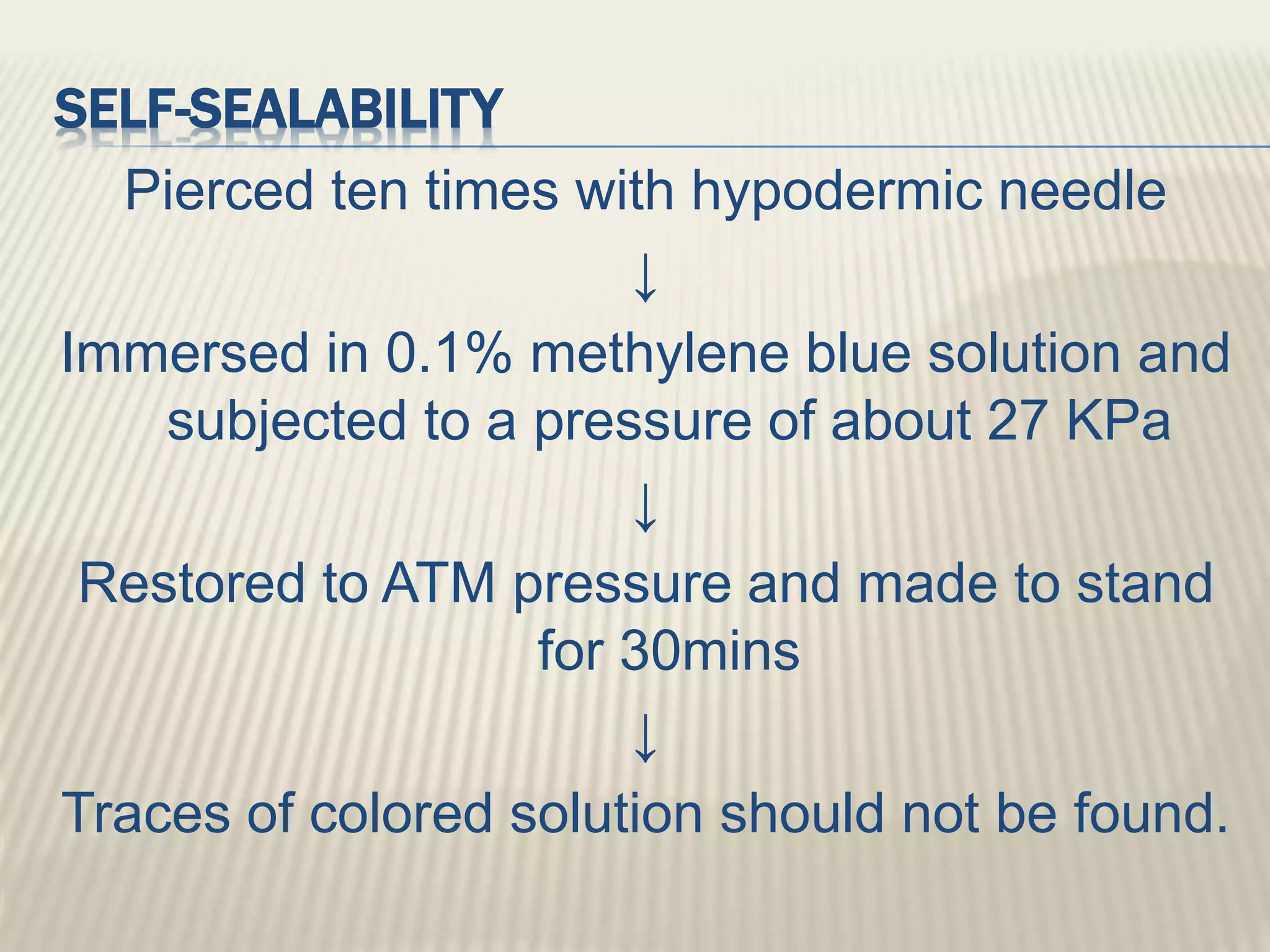
This document discusses quality control tests for pharmaceutical packing materials. It describes the different types of primary, secondary, and tertiary packing materials used in the pharmaceutical industry. It focuses on quality control tests for various container materials like glass and plastic. Some key tests discussed for glass containers include chemical resistance testing, hydrolytic resistance testing, surface etching testing, and light transmission testing. For plastic containers, leakage testing, collapsibility testing, clarity of aqueous extract testing, and non-volatile residue testing are described. The document provides details on procedures and acceptance criteria for many of these important quality control tests.