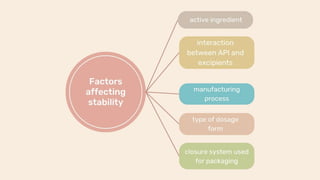
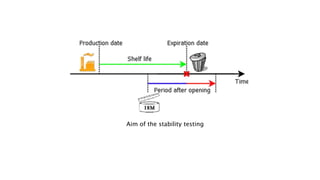
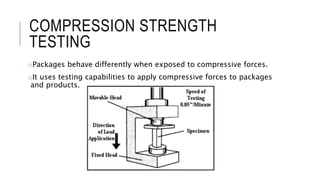
The document discusses stability testing of packaging materials. Stability testing aims to determine how the quality of packaging varies over time under different environmental conditions, establish shelf-life, and determine suitable storage conditions. It also ensures the packaging can withstand forces involved in compression, distribution simulation, and maintains integrity. The document outlines protocols for stability testing according to ICH guidelines and different tests conducted including compression strength, distribution simulation, package strength, and package integrity testing.