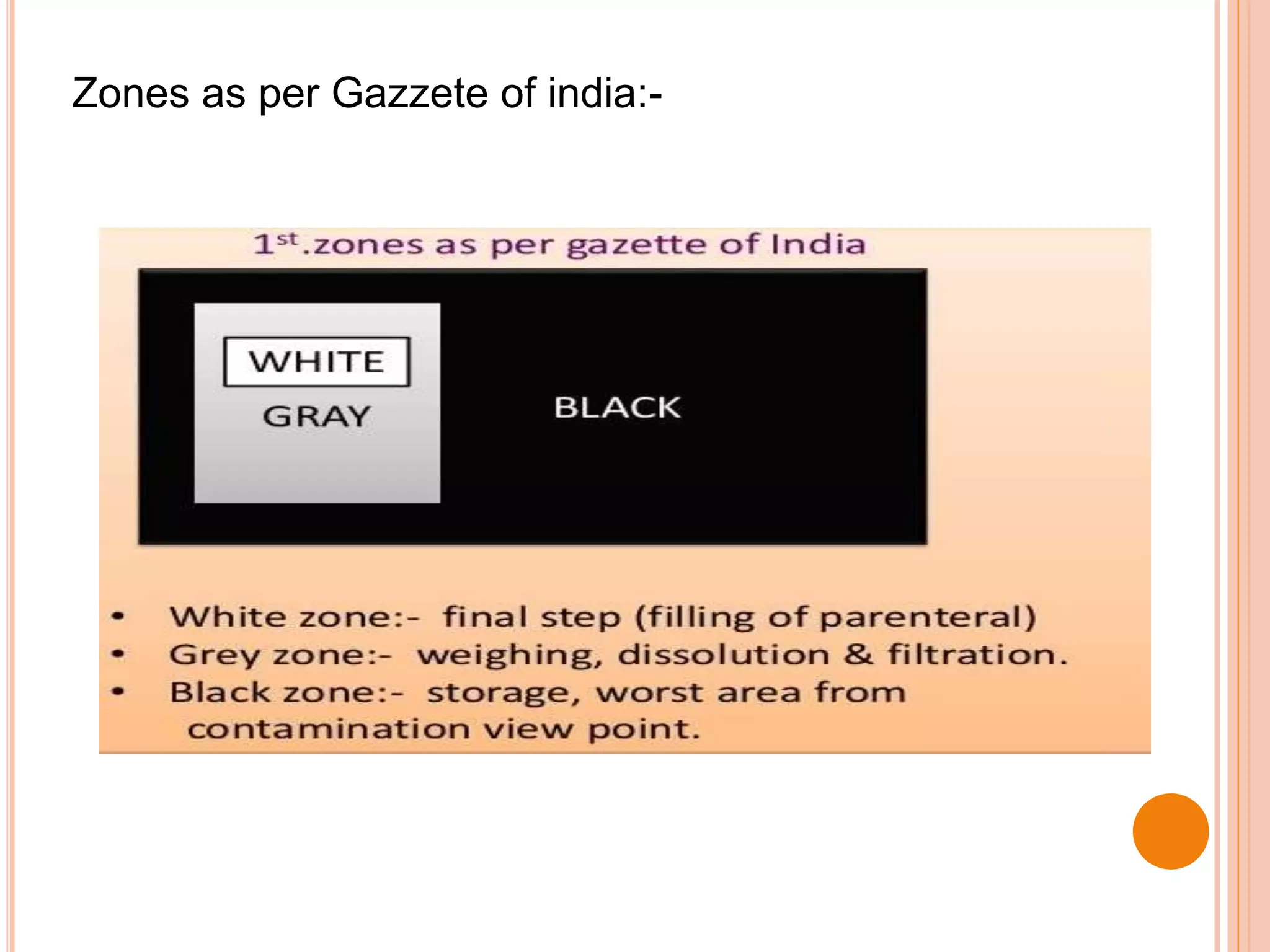
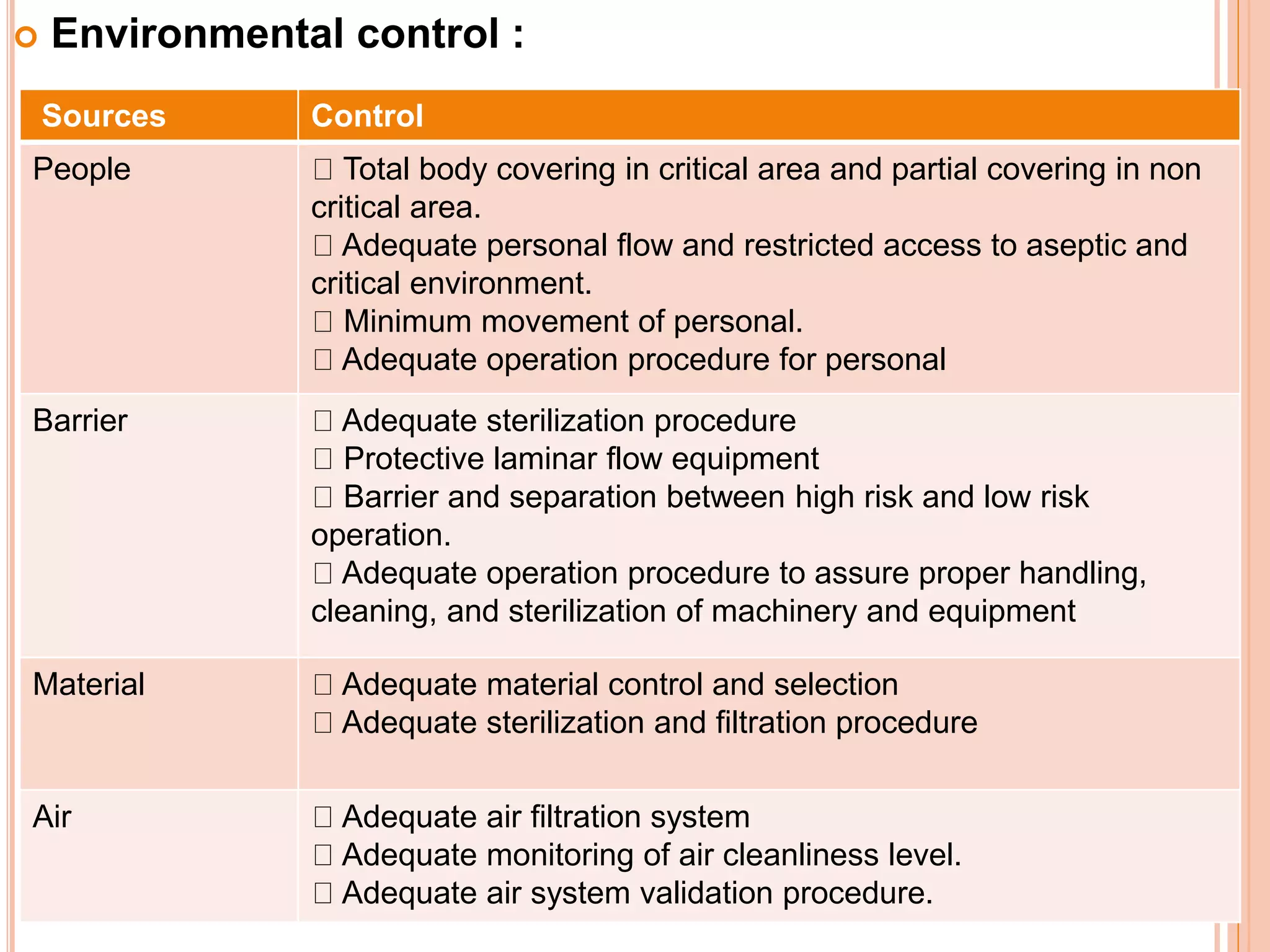
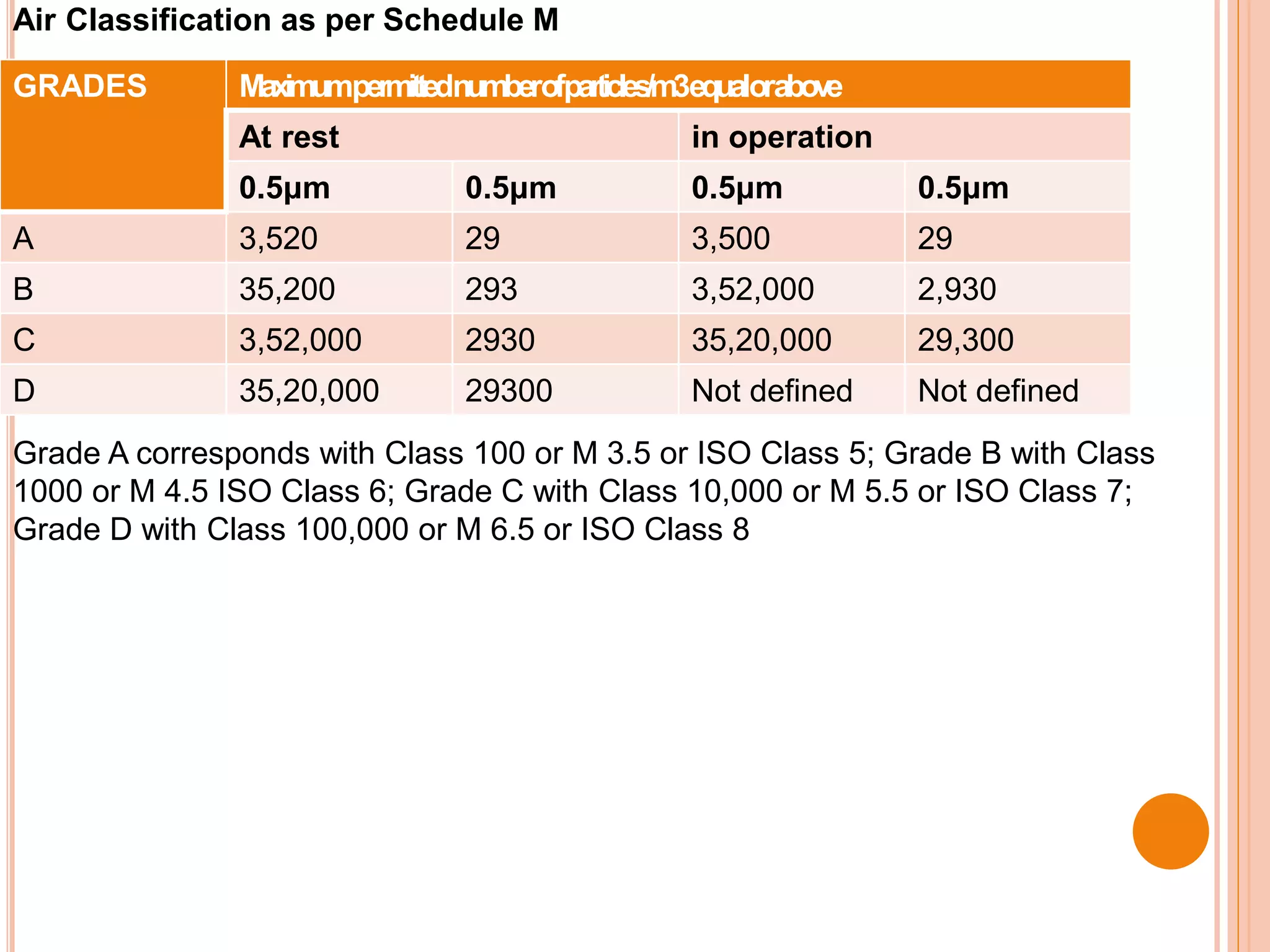
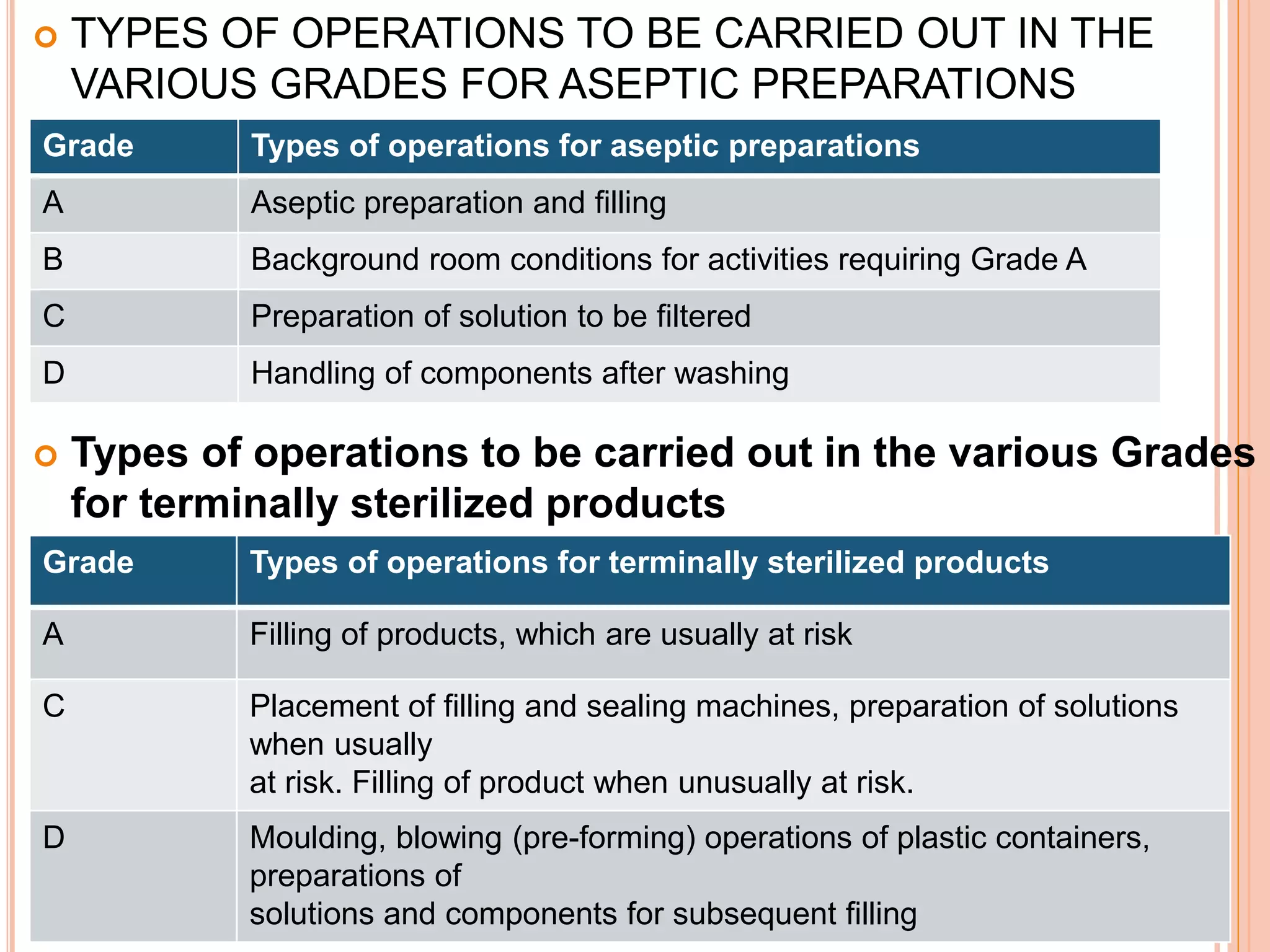
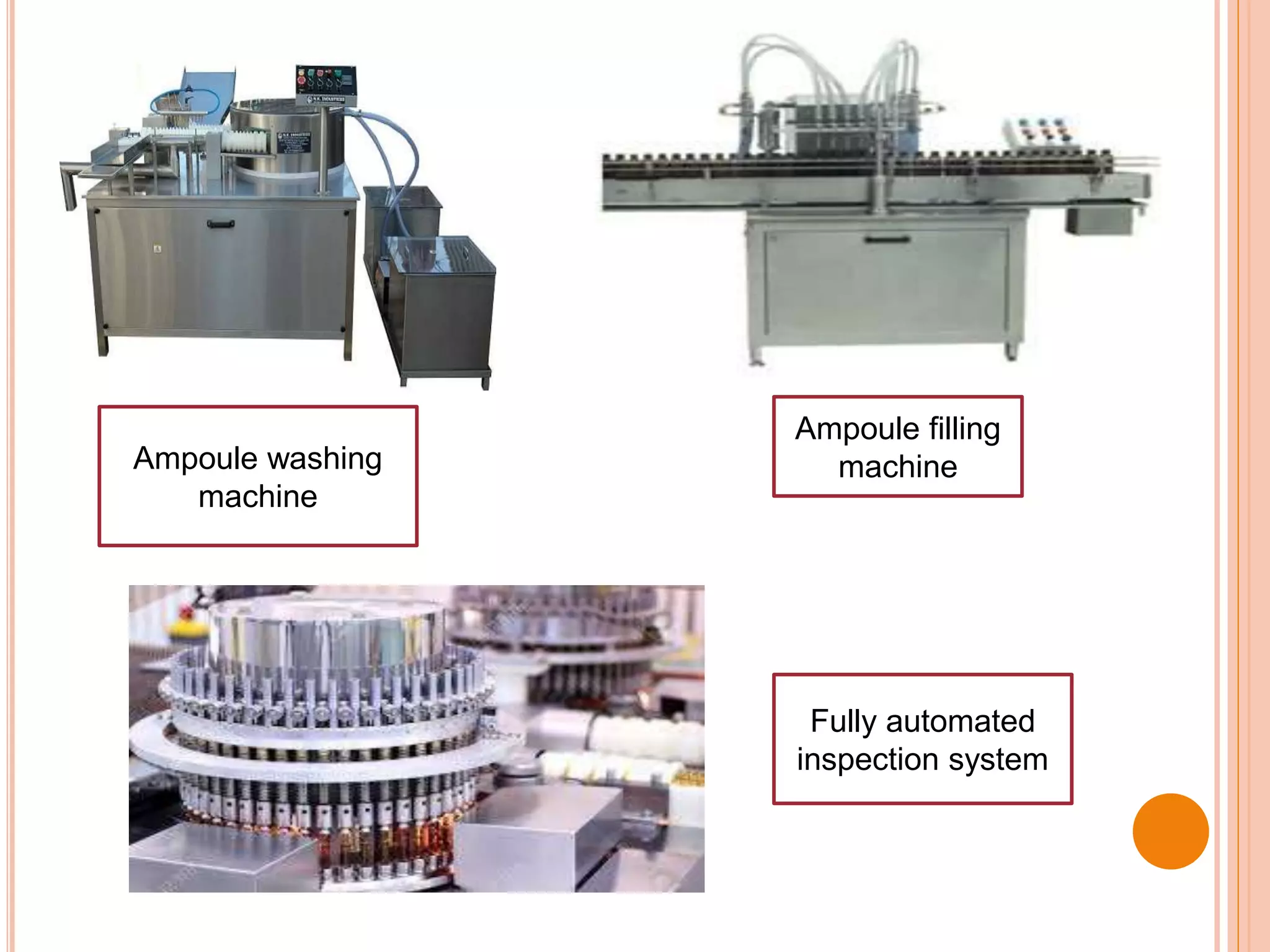
This document discusses the formulation, manufacturing, and quality control of small volume parenterals (SVPs). It defines SVPs as injections packaged in containers of 100ml or less. The key points are:
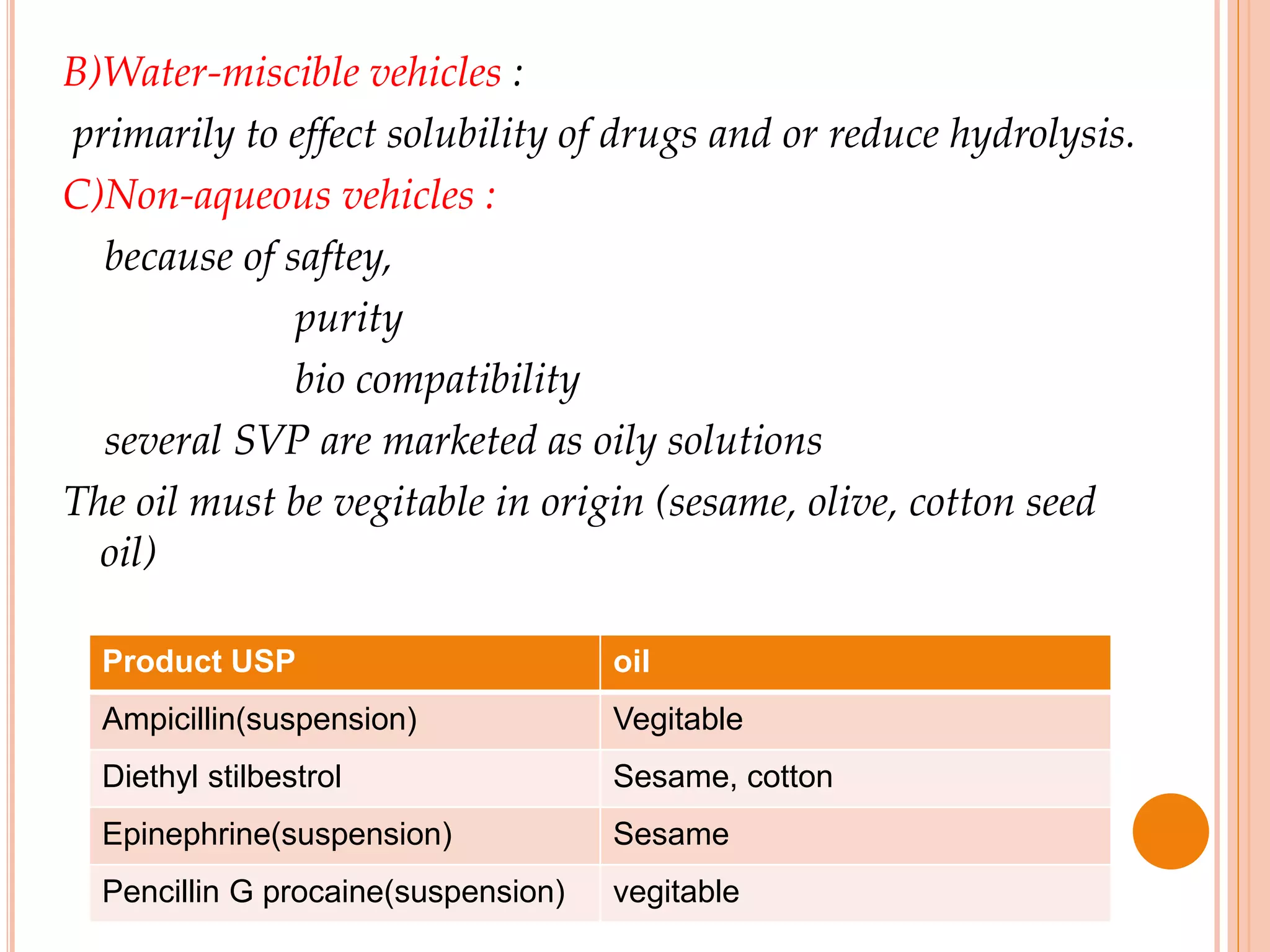
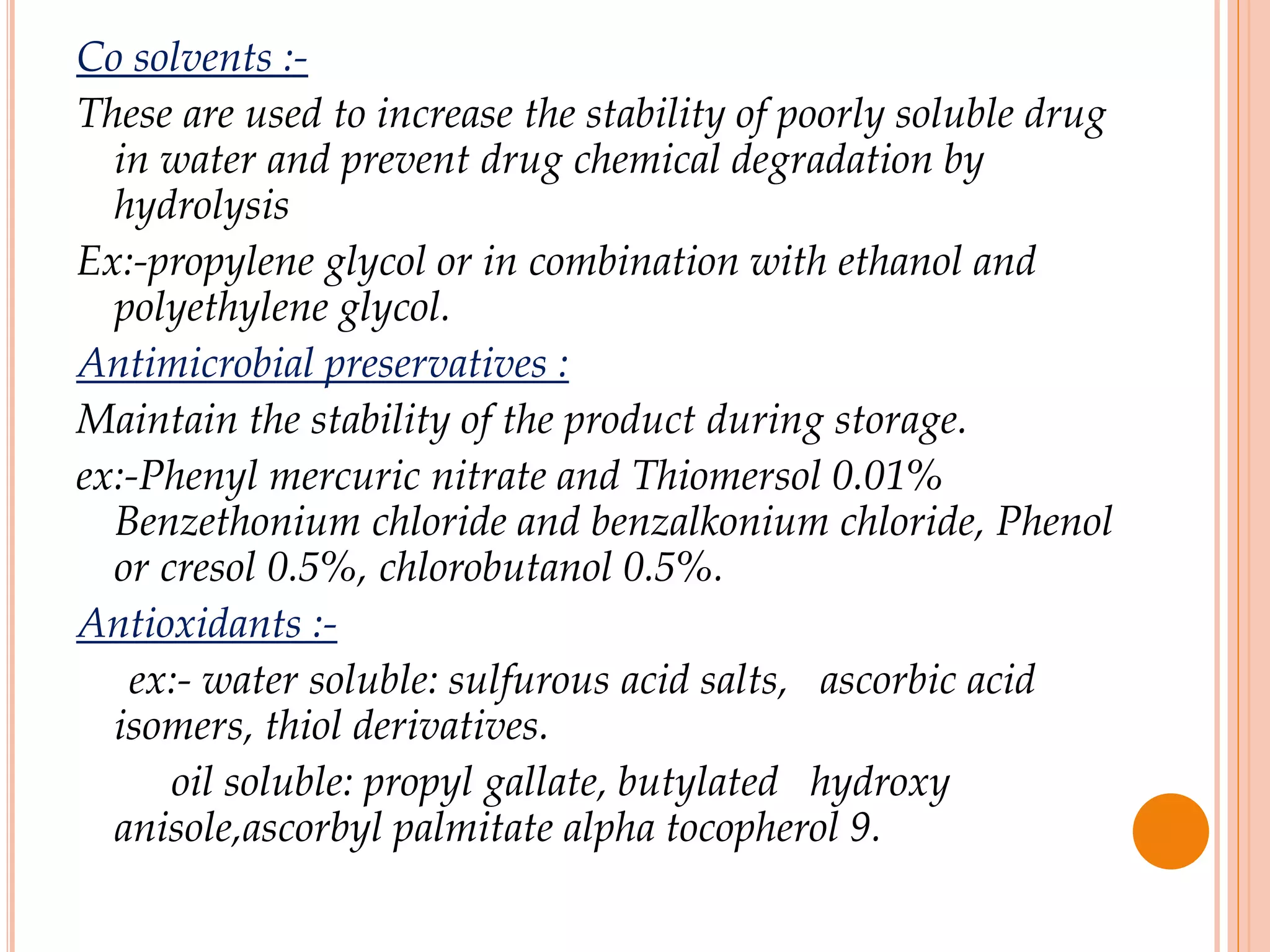
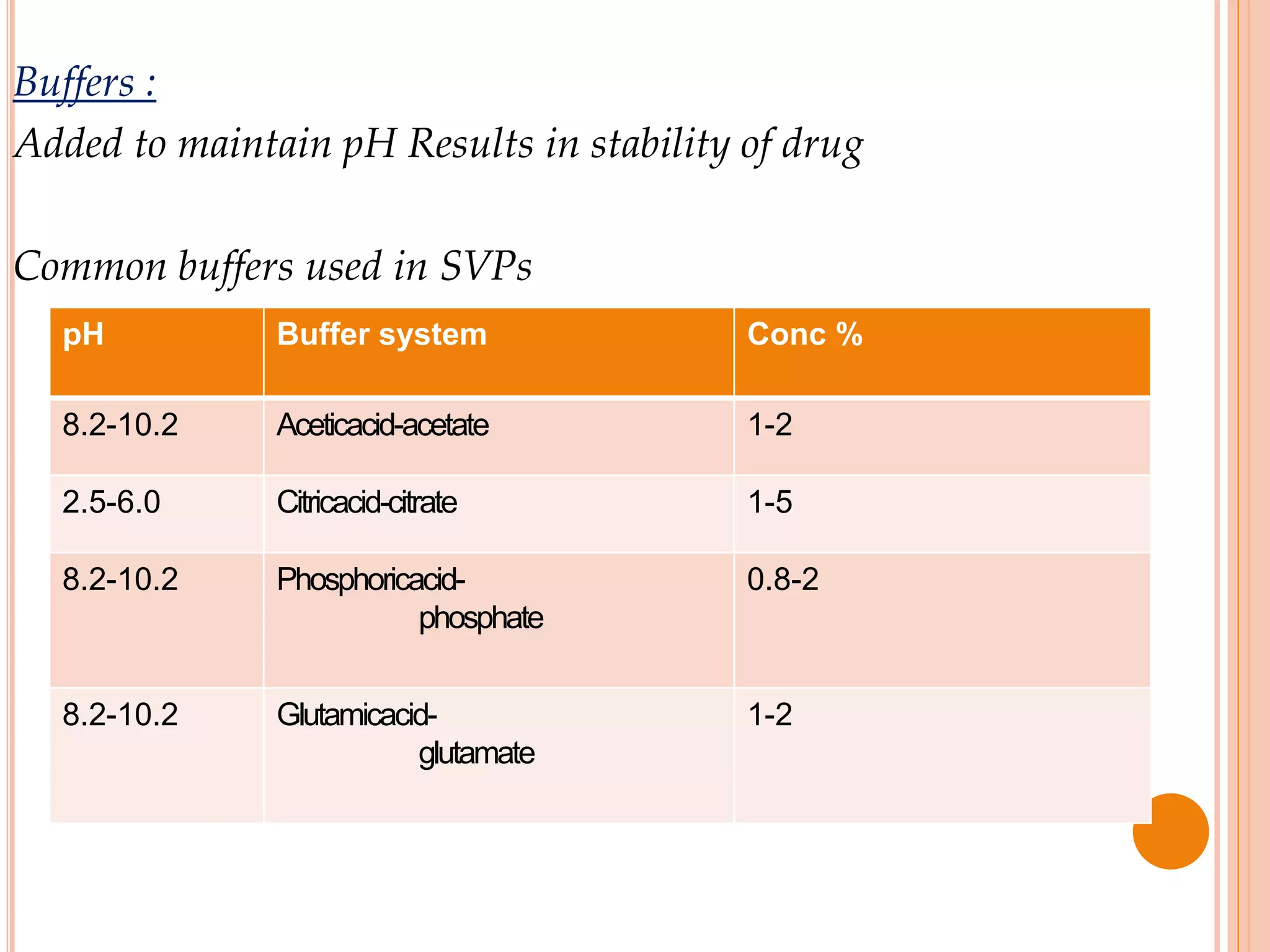
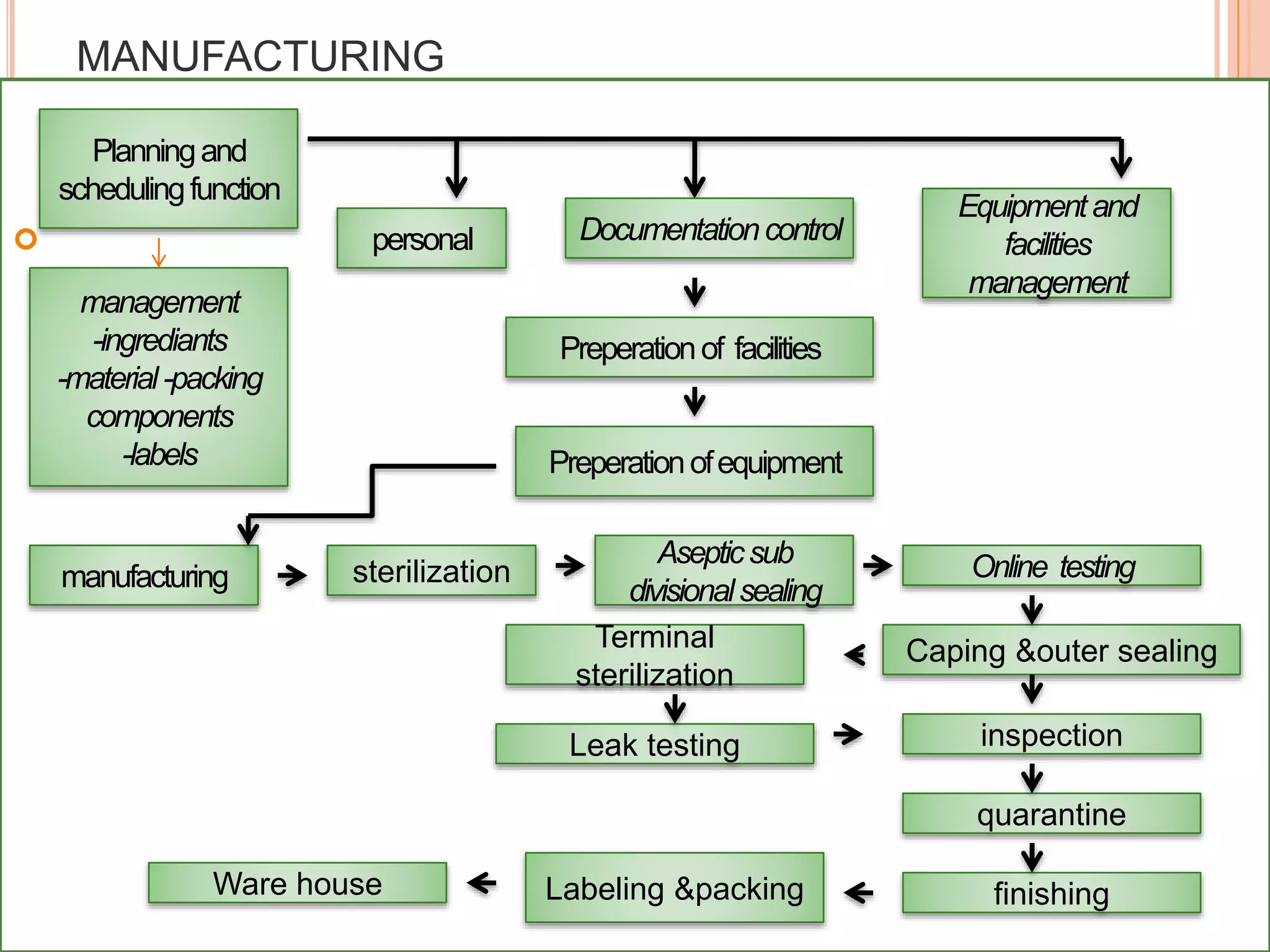
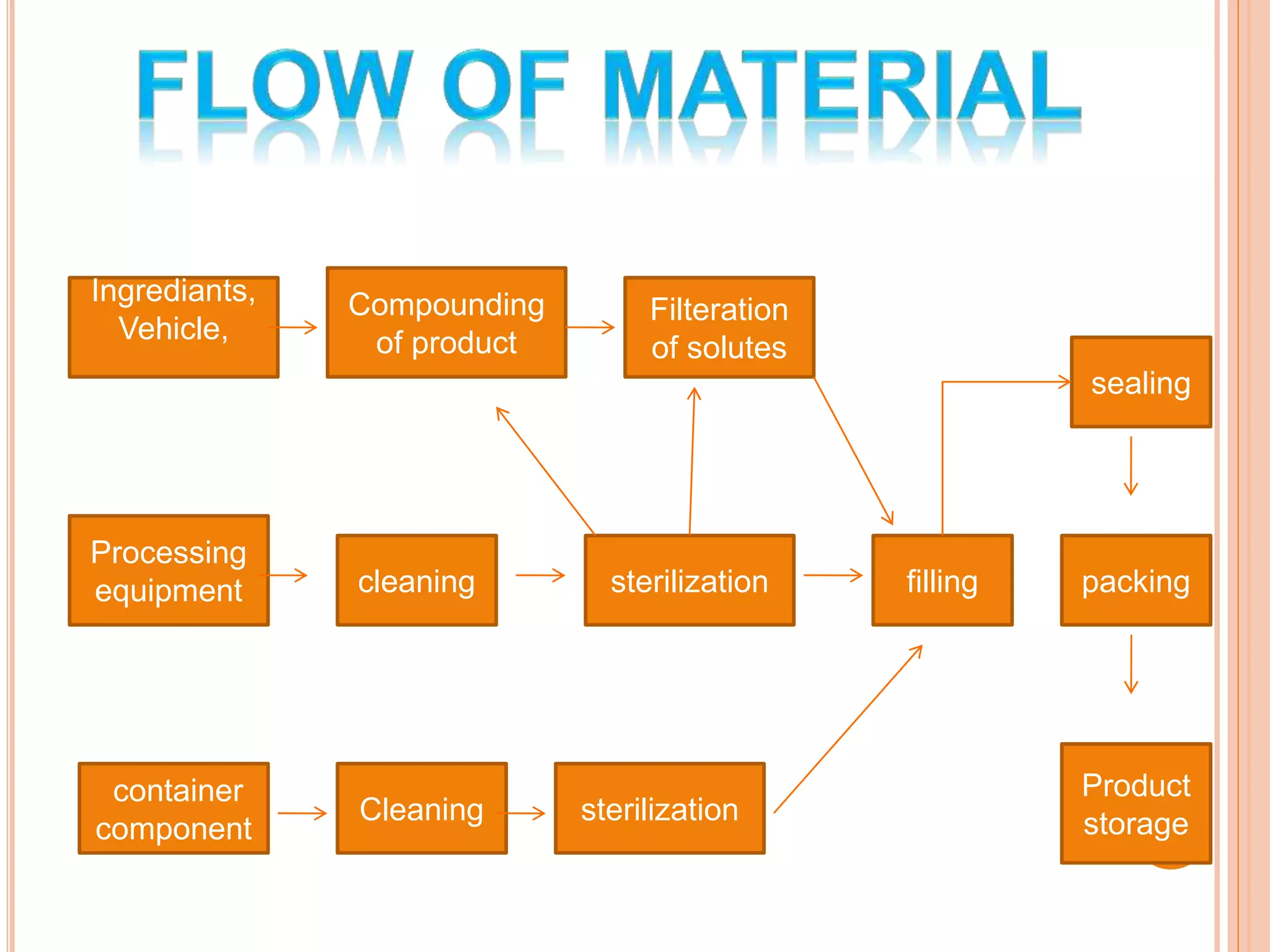
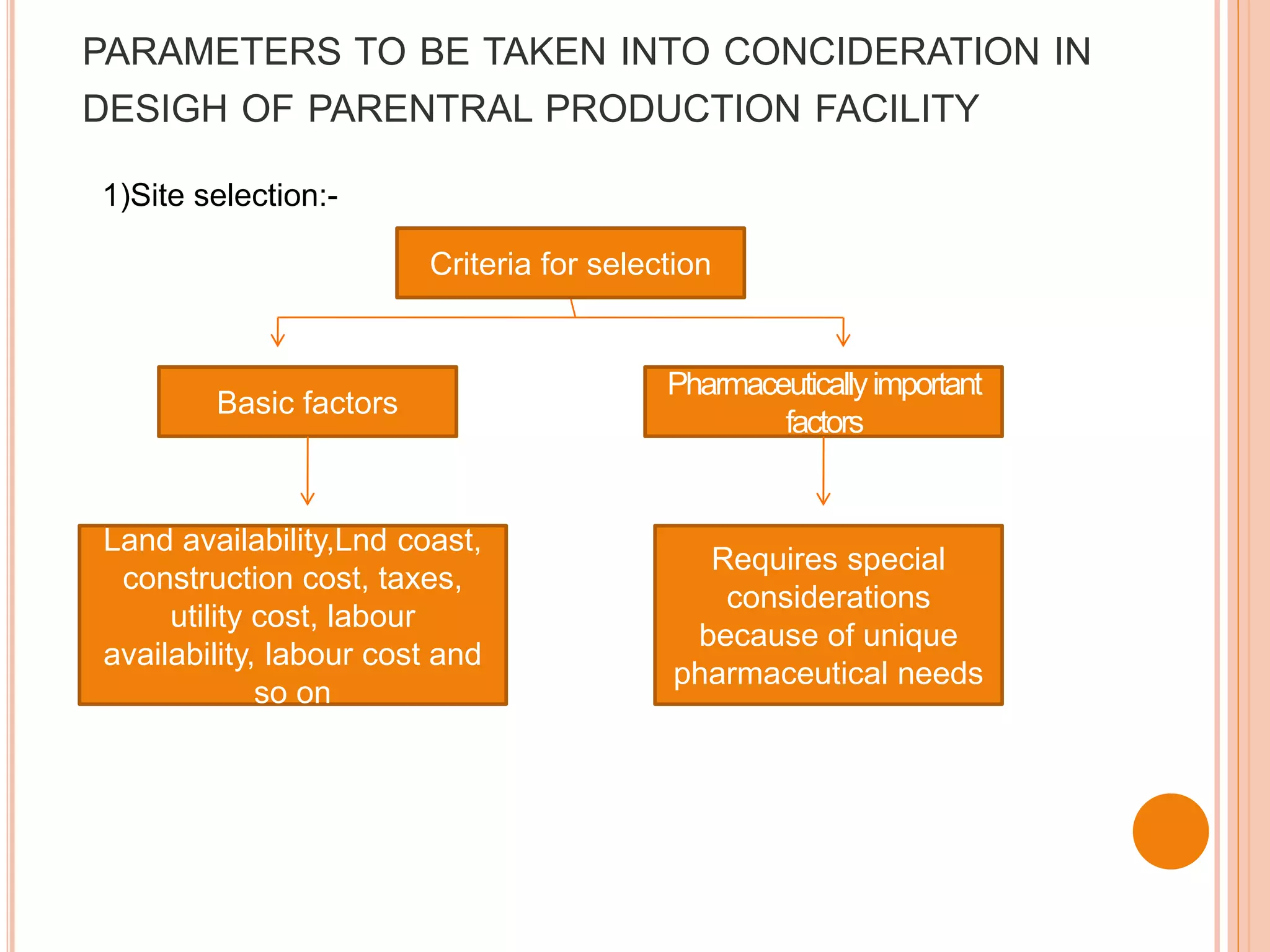
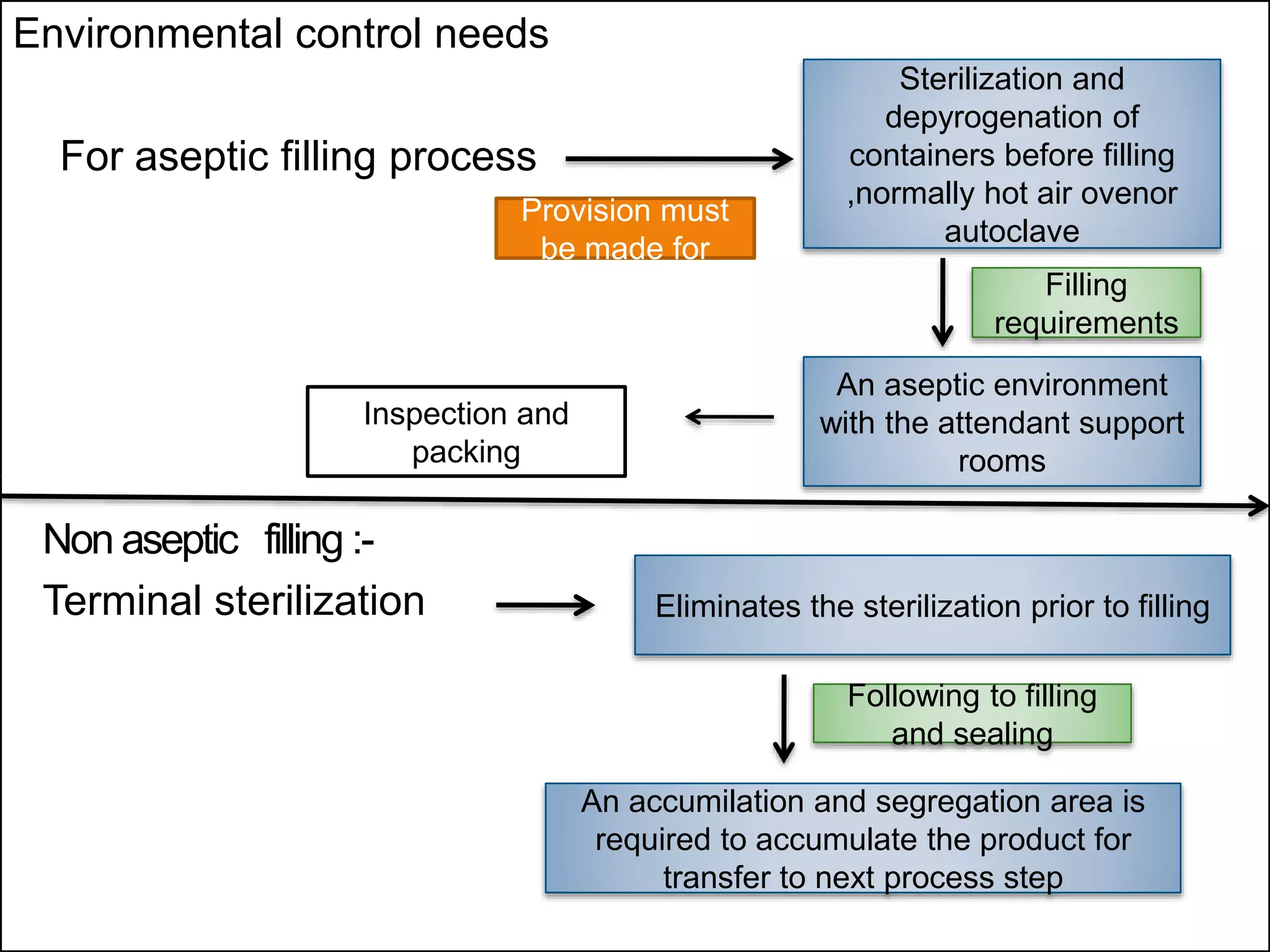
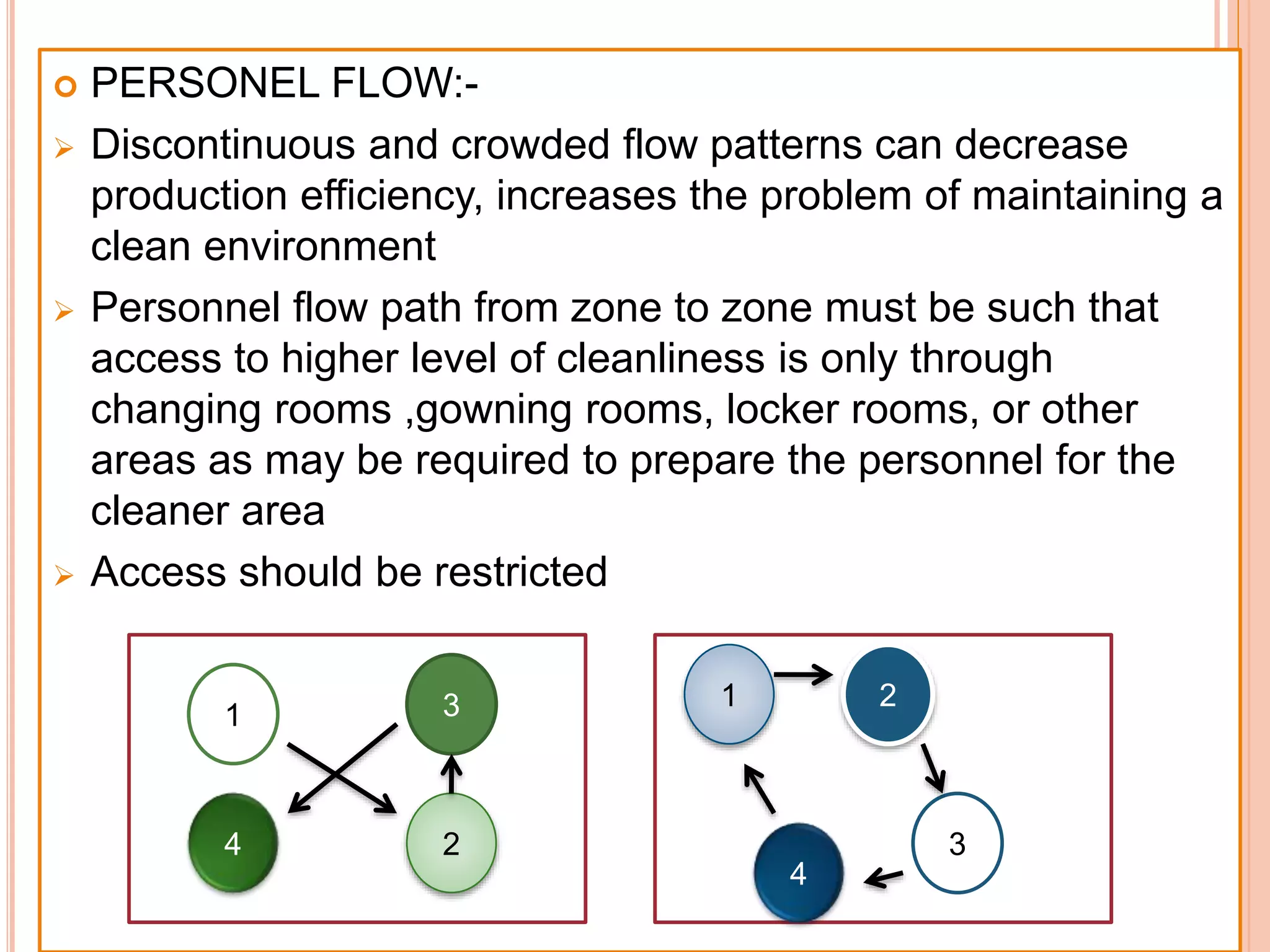
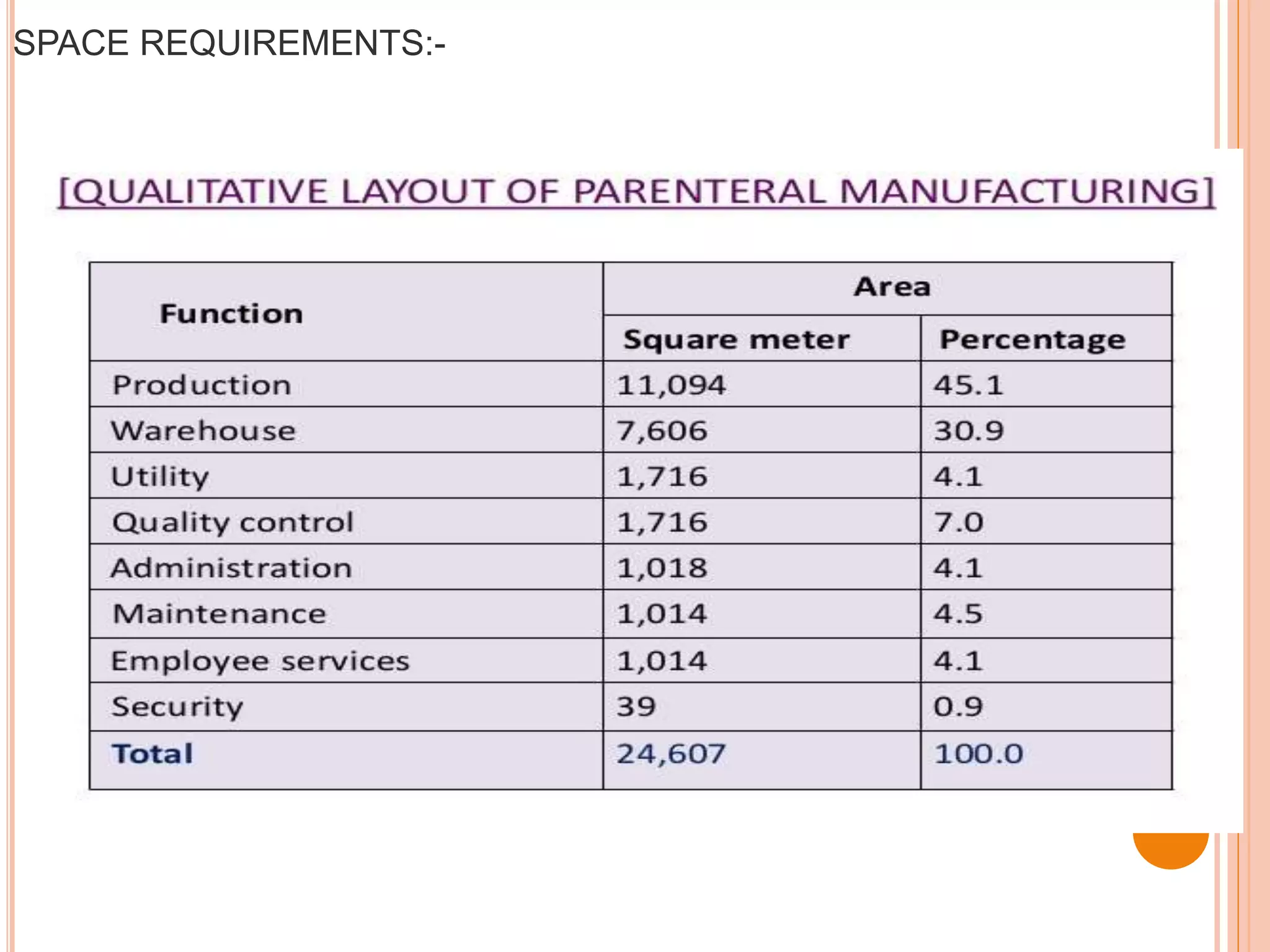
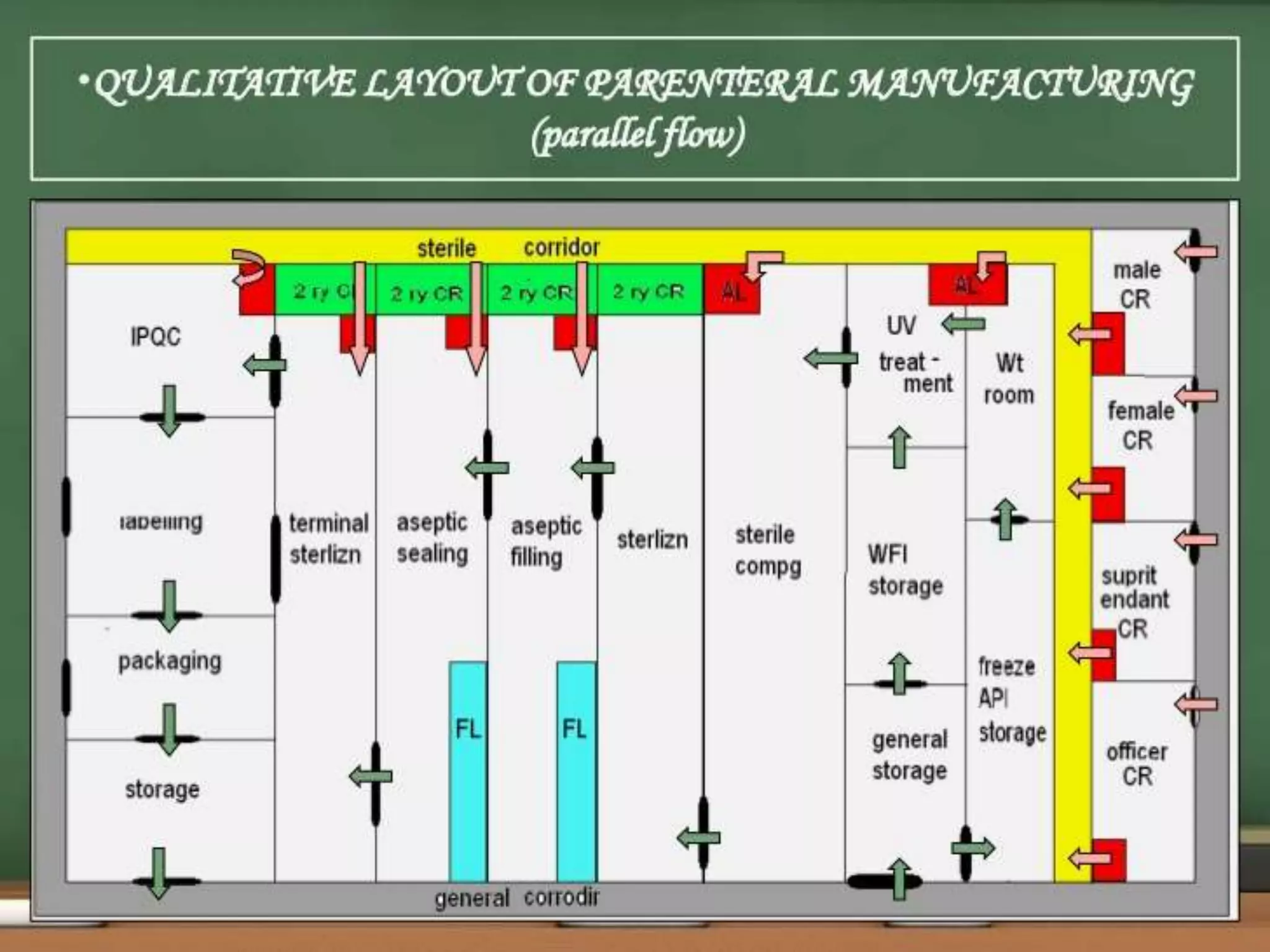
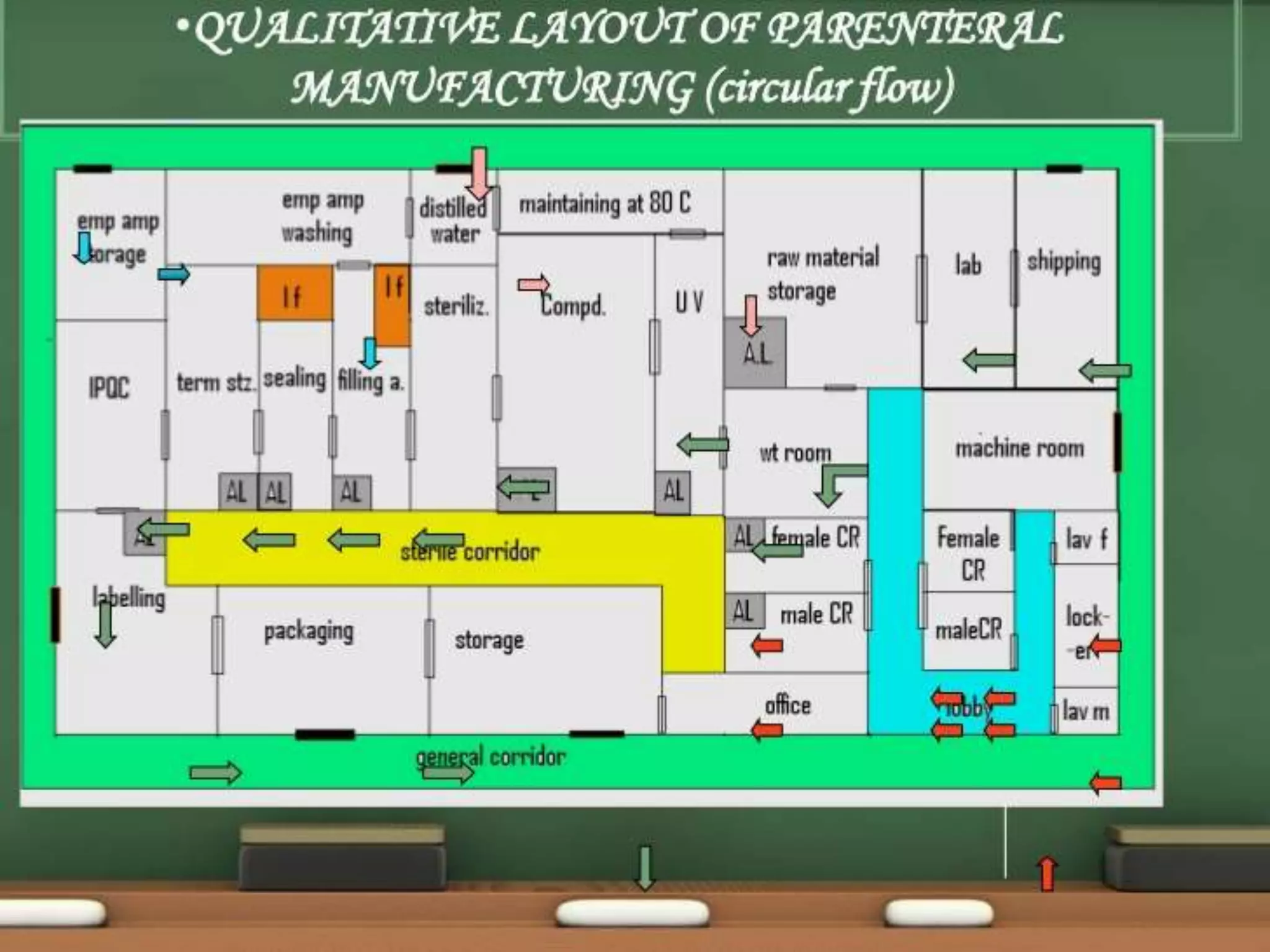
SVPs can be single dose ampoules, multiple dose vials, or prefilled syringes. Their formulations typically include an aqueous, water-miscible, or non-aqueous vehicle along with buffers, preservatives, antioxidants, and tonicity adjusters. Manufacturing involves preparation, sterilization, filling, and sealing in clean rooms with proper air filtration and personnel flow. Quality control tests include particulate matter, leakage, clarity, sterility, pyrogen and endotoxin tests.