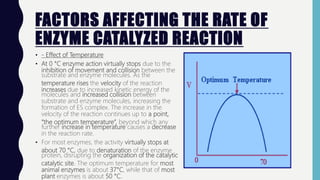
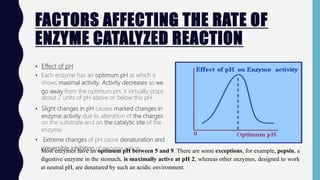
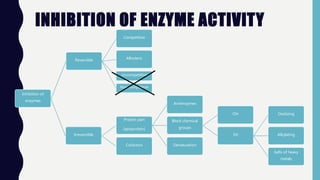
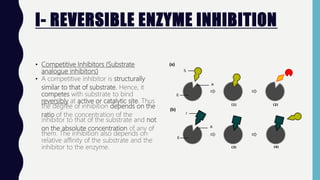
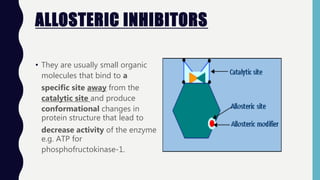
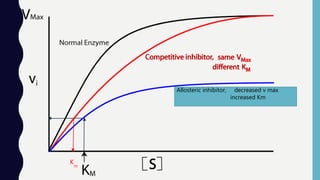
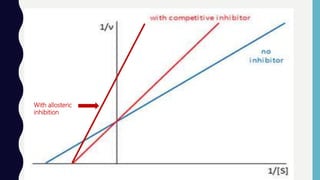
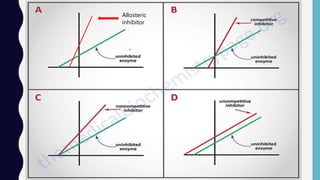
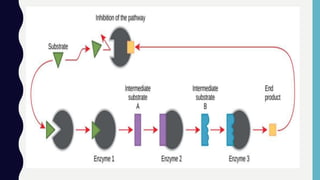
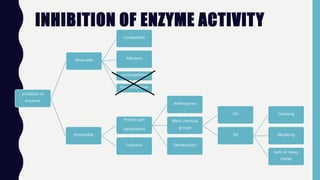
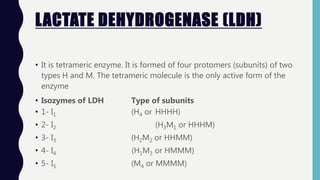
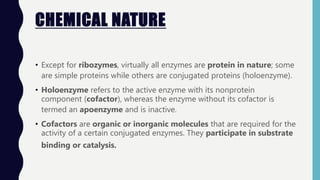
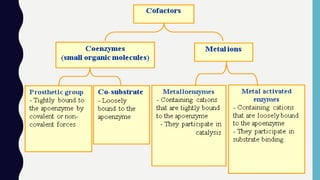
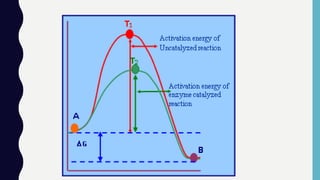
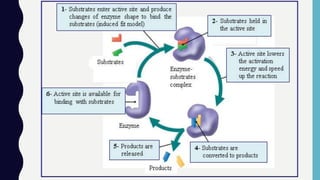
Enzymes are biocatalysts, primarily proteins, that accelerate biochemical reactions and exhibit high specificity for substrates. Their activity is influenced by factors such as substrate concentration, temperature, pH, and the presence of cofactors, with inhibition mechanisms further affecting their function. Regulation of enzyme activity involves adjusting enzyme levels, utilizing allosteric modifiers, and covalent modifications such as phosphorylation.






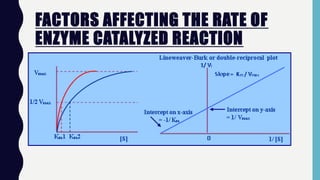
![FACTORS AFFECTING THE RATE OF
ENZYME CATALYZED REACTION
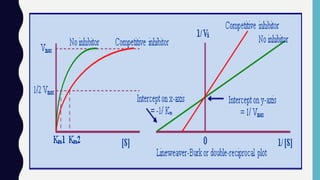
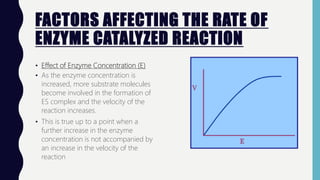
• Effect of Substrate Concentration [S]
• The substrate concentration that
produces half the maximal velocity is
termed Michaelis constant or Km .
• Smaller Km reflects higher affinity of
the enzyme for its substrate](https://image.slidesharecdn.com/enzymes2019-191203115352/85/Enzymes-2019-11-320.jpg)
![FACTORS AFFECTING THE RATE OF
ENZYME CATALYZED REACTION
• Effect of Concentration of Cofactors
[C]
• The velocity of the reaction will be
directly proportional to the
concentration of the cofactor. This is
true up to the point when each
enzyme molecule is associated with
the cofactor required. At this point
increase in cofactor concentration will
not increase the velocity of the
reaction](https://image.slidesharecdn.com/enzymes2019-191203115352/85/Enzymes-2019-14-320.jpg)