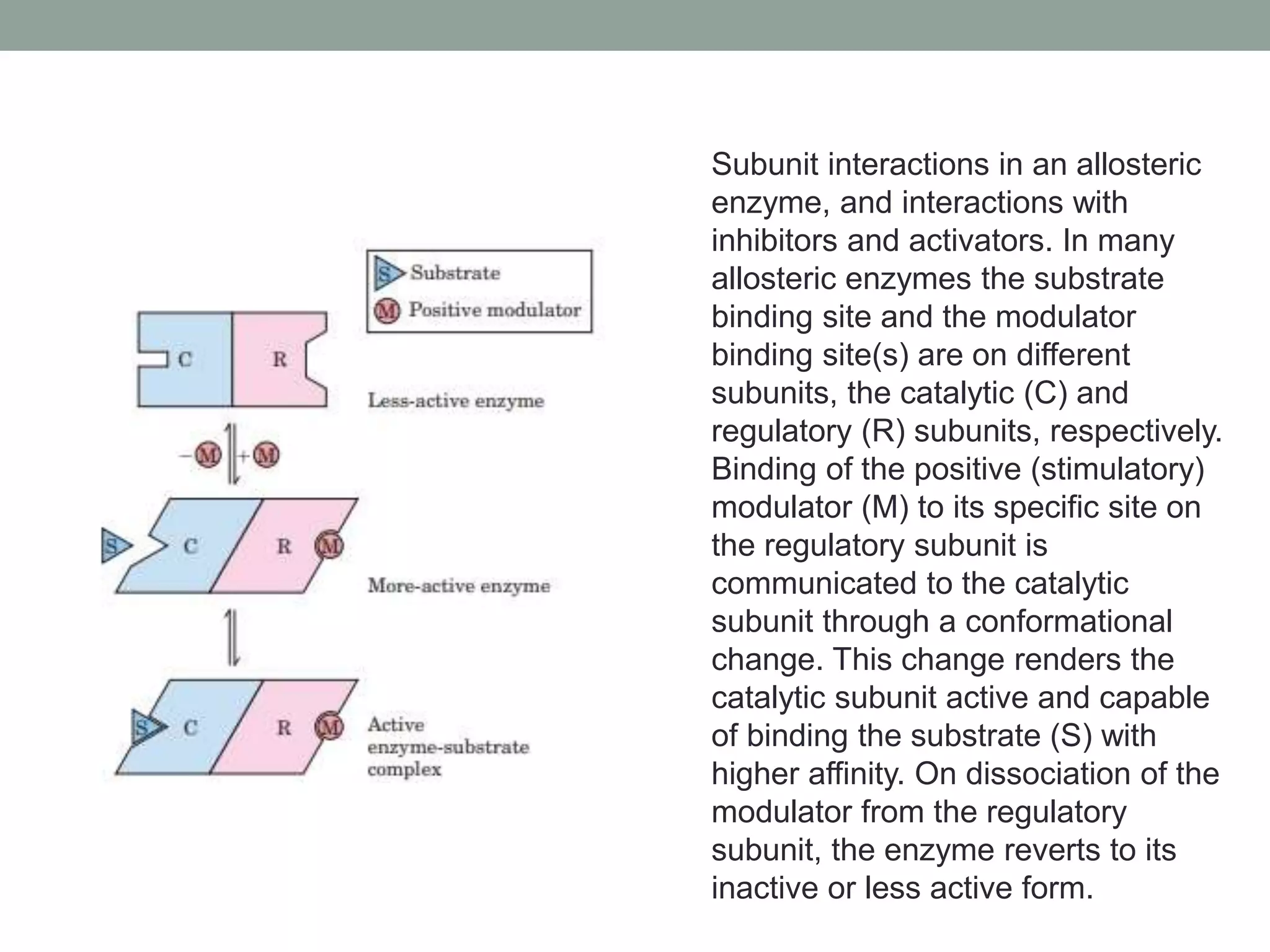
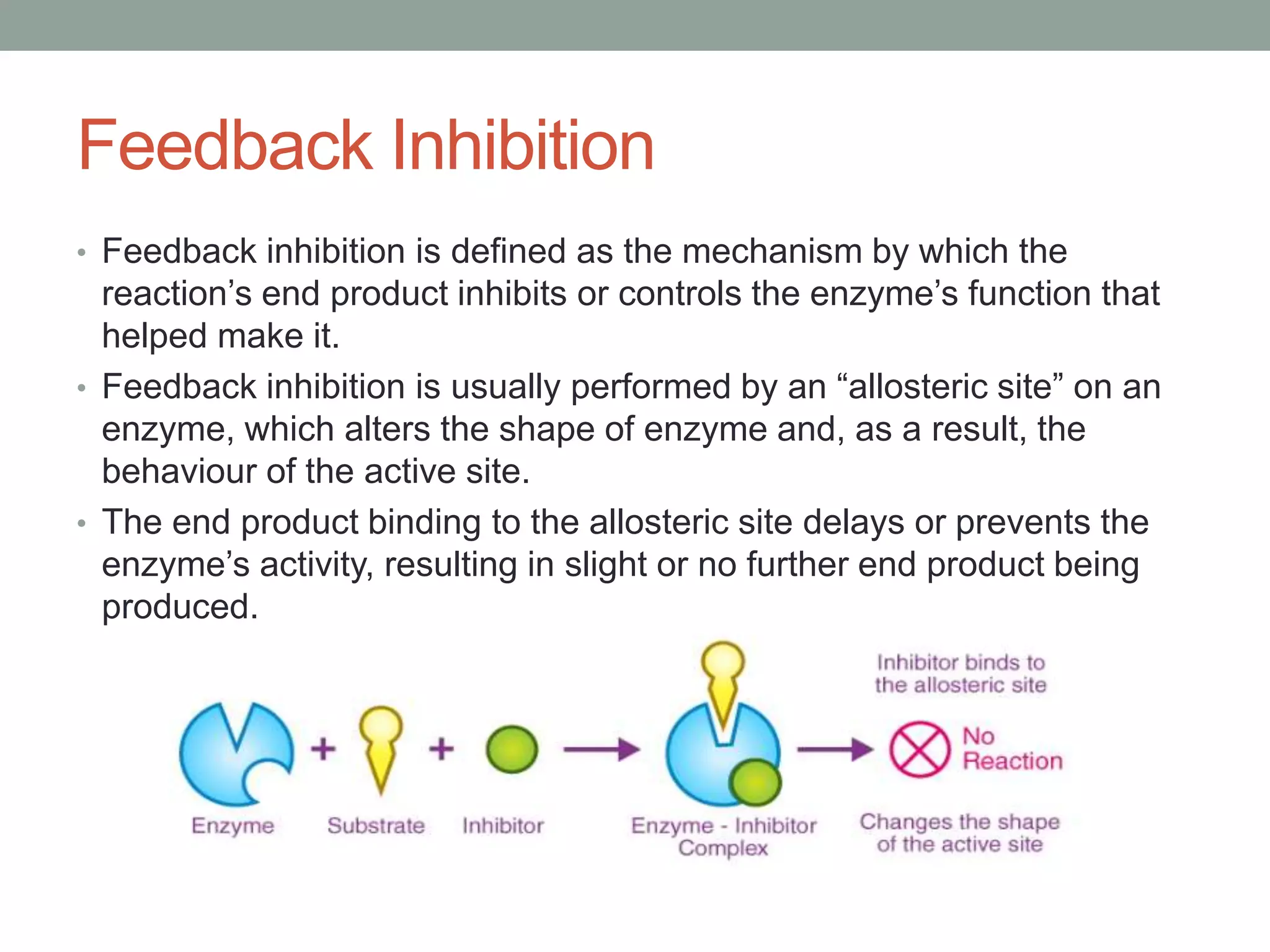
This document discusses allosteric regulation of enzymes. It defines allosteric regulation as the regulation of an enzyme by binding an effector molecule at a site other than the active site. Effectors that increase enzyme activity are called positive allosteric effectors, while those that decrease activity are negative effectors. Allosteric enzymes can exist in more or less active conformations depending on the binding of effectors. Feedback inhibition is also described, where the end product of a pathway inhibits an earlier regulatory enzyme. Allosteric enzymes do not always follow Michaelis-Menten kinetics and can display sigmoidal substrate binding curves.








![Allosteric enzymes show relationships between V0 and [S]
that differ from Michaelis-Menten kinetics.
They do exhibit saturation with the substrate when [S] is
sufficiently high, but for some allosteric enzymes, plots of
V0 versus [S] produce a sigmoid saturation curve, rather
than the hyperbolic curve typical of nonregulatory enzymes.
On the sigmoid saturation curve we can find a value of [S]
at which V0 is half-maximal, but we cannot refer to it with
the designation Km, because the enzyme does not follow
the hyperbolic MichaelisMenten relationship.
Instead, the symbol [S]0.5 or K0.5 is often used to
represent the substrate concentration giving half-maximal
velocity of the reaction catalyzed by an allosteric enzyme
The Kinetic properties of allosteric enzymes diverge
from Michaelis Menton kinetics](https://image.slidesharecdn.com/regulationofenzymes-230725072149-ee2ec1be/75/Regulation-of-Enzymes-pptx-12-2048.jpg)
![Kinetic properties of homotropic allosteric
enzymes
• Homotropic allosteric enzymes generally are multisubunit
proteins and, as noted earlier, the same binding site on each
subunit functions as both the active site and the regulatory site.
• Most commonly, the substrate acts as a positive modulator (an
activator), because the subunits act cooperatively.
• The binding of one molecule of substrate to one binding site
alters the enzyme’s conformation and enhances the binding of
subsequent substrate molecules.
• This accounts for the sigmoid rather than hyperbolic change in
V0 with increasing [S]. One characteristic of sigmoid kinetics is
that small changes in the concentration of a modulator can be
associated with large changes in activity.](https://image.slidesharecdn.com/regulationofenzymes-230725072149-ee2ec1be/75/Regulation-of-Enzymes-pptx-13-2048.jpg)

![Kinetic properties of homotropic allosteric
enzymes
• For heterotropic allosteric enzymes:
• An activator may cause the curve to become more nearly
hyperbolic, with a decrease in K0.5 but no change in
Vmax, resulting in an increased reaction velocity at a fixed
substrate concentration.
• V0 is higher for any value of [S].](https://image.slidesharecdn.com/regulationofenzymes-230725072149-ee2ec1be/75/Regulation-of-Enzymes-pptx-15-2048.jpg)


