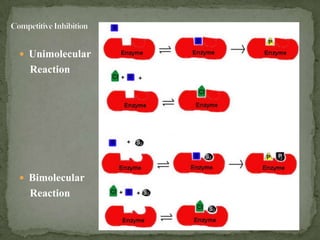
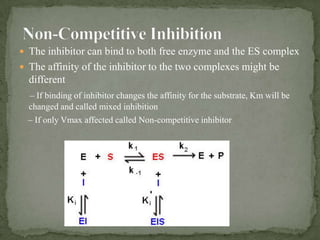
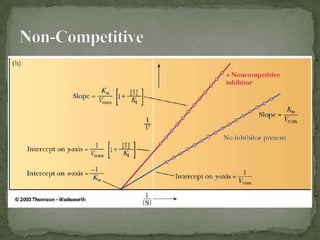
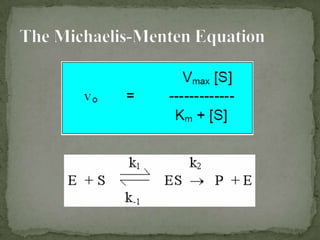
This document discusses enzyme kinetics and inhibition. It describes how enzymes exert kinetic control over metabolic pathways and reactions. It aims to determine the maximum velocity, substrate affinity, and inhibitor affinity of enzymes. This can provide information about metabolic pathway flow, substrate utilization, and how to manipulate metabolic events. The document also discusses Michaelis-Menten kinetics, Lineweaver-Burk plots, competitive and non-competitive inhibition, and how inhibitors can be used to study enzyme mechanism and regulation.

![ when [S]= KM, the equation reduces to
when [S] >> KM, the equation reduces to
when [S] << KM, the equation reduces to](https://image.slidesharecdn.com/enzymekineticsandenhibition-130406072209-phpapp02/85/Enzyme-kinetics-and-inhibition-3-320.jpg)
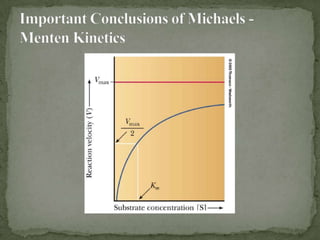
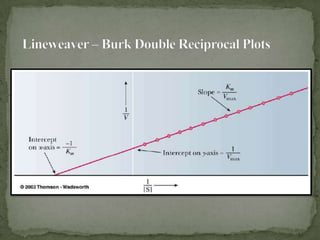
![ It is difficult to determine Vmax experimentally
The equation for a hyperbola can be transformed into
the equation for a straight line by taking the reciprocal
of each side
The formula for a straight line is y = mx + b
A plot of 1/V versus 1/[S] will give a straight line with
slope of KM/Vmax and y intercept of 1/Vmax
Such a plot is known as a Lineweaver-Burk double
reciprocal plot](https://image.slidesharecdn.com/enzymekineticsandenhibition-130406072209-phpapp02/85/Enzyme-kinetics-and-inhibition-5-320.jpg)