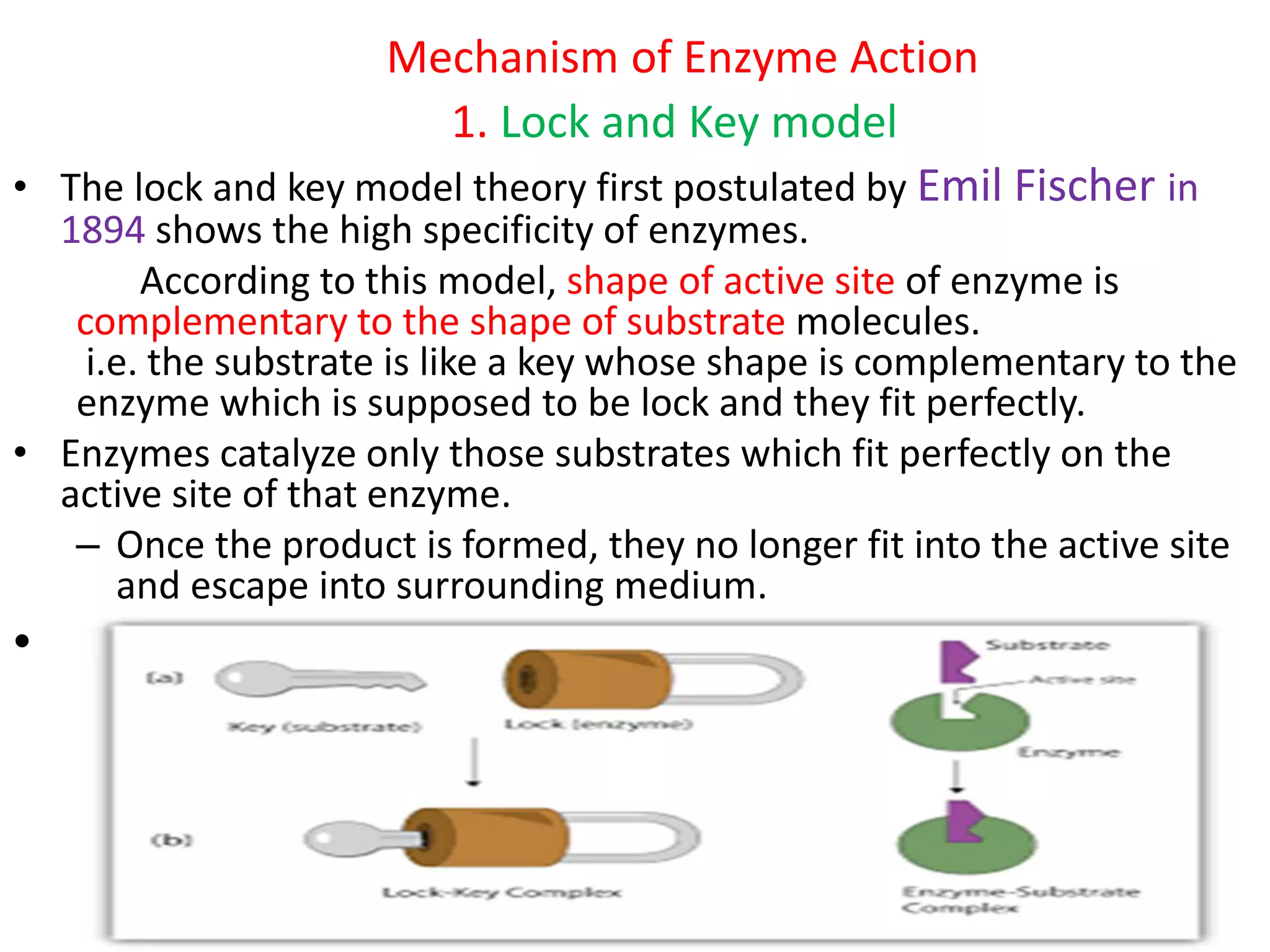
Enzymes are proteins that act as biological catalysts and accelerate chemical reactions. They contain an active site that binds specifically to substrate molecules. Originally, it was thought that the active site and substrate were perfectly complementary in shape, as described by the lock-and-key model. However, the induced fit model proposes that the active site is flexible and changes conformation upon substrate binding to optimize the enzyme-substrate interaction. Specifically, when a substrate interacts with the active site, it induces a change in the active site's shape to better fit the substrate, forming a stable complex and lowering the energy of activation for the chemical reaction.