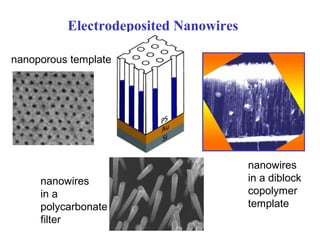
Electroplating is a process that uses electrical current to deposit metal ions from a solution onto an electrode. It allows precise control over deposition rate by adjusting current or voltage. Electroplating can be used to make nanostructures by depositing metal into a porous template, such as a polycarbonate filter or diblock copolymer. The amount deposited is directly proportional to the current over time. Electroplating is a simple way to make nanowires for applications such as data storage elements, where magnetic nanowires can store data through magnetization direction.