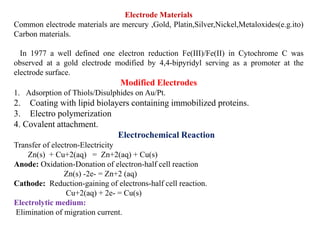
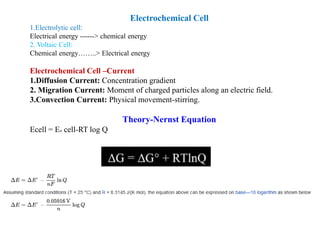
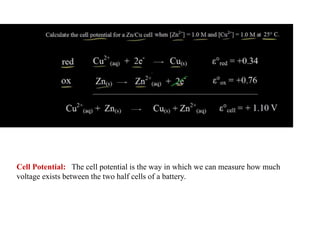
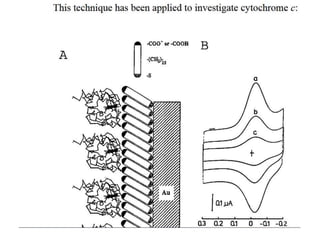
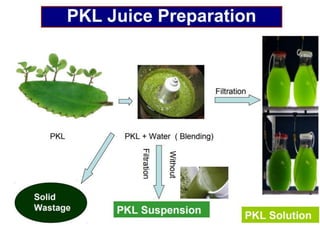
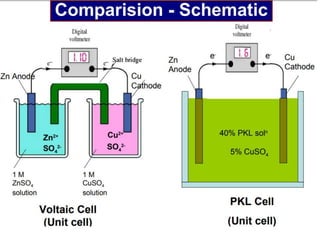
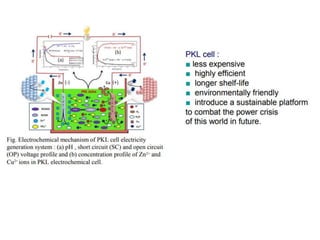
1. The document discusses electrochemistry and its application to proteins. It examines how electrochemical parameters like pH, potential difference, and electrode material can be varied to selectively modify amino acids in proteins.
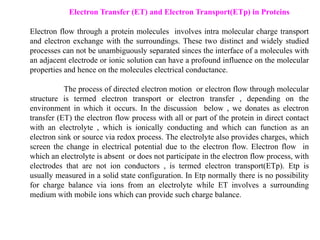
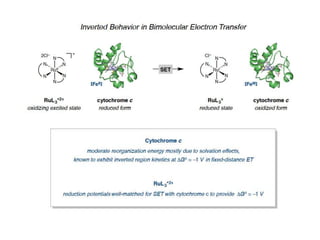
2. It also discusses electron transfer reactions in proteins, noting that proteins contain redox-active groups that facilitate intramolecular charge transport and electron exchange. Special orientations of the protein on electrode surfaces and use of mediators are often required.
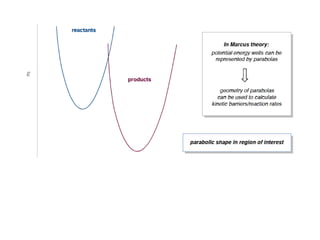
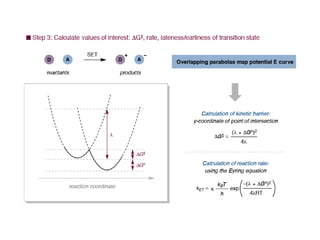
3. The kinetics of electron transfer in proteins is slow due to diffusion limitations and the need to overcome reorganization energy for electron transfer to occur. Marcus theory provides an explanation for electron transfer rates.