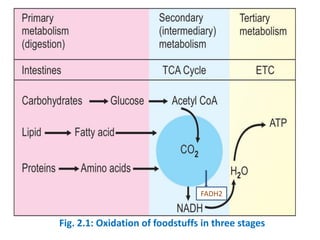
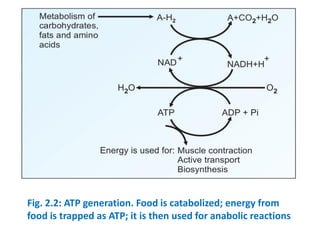
The document discusses biological oxidation and the electron transport chain. It covers topics like the stages of foodstuff oxidation, redox potentials, enzymes and co-enzymes in biological oxidation, high energy compounds, the organization of the electron transport chain, oxidative phosphorylation, and ATP synthesis. It notes that food is broken down and oxidized to generate reducing equivalents like NADH and FADH2, which then enter the electron transport chain to release energy that is used to synthesize ATP through oxidative phosphorylation.