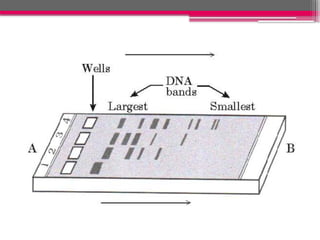
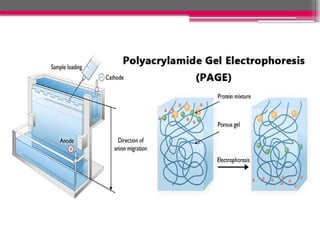
Electrophoresis is a laboratory technique used to separate charged molecules based on their size and charge by applying an electric field. The process involves sample preparation, loading into a gel matrix, application of an electric current, separation of molecules, and visualization. It has various applications in nutrition, including protein analysis, genetic studies, and quality control, making it essential for understanding nutritional content and food safety.