1) Heat is a form of energy that is transferred between objects at different temperatures. Heat transfer occurs when samples are in contact and energy moves from the higher temperature sample to the lower temperature sample.
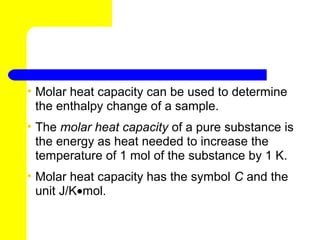
2) Enthalpy is a measurement of the total energy of a system and includes both the internal energy and pressure-volume work term. The enthalpy change of a reaction can be determined from standard enthalpies of formation values.
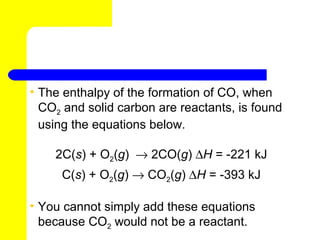
3) Hess's law states that the enthalpy change of an overall chemical process is equal to the sum of the enthalpy changes of the individual steps in that process.