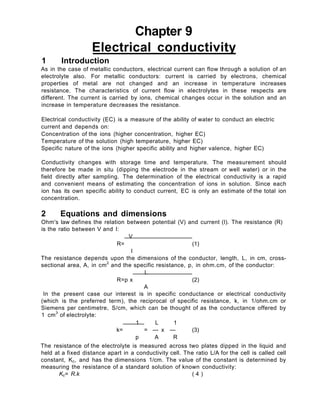
The document provides information about measuring electrical conductivity:
- Electrical conductivity is a measure of a solution's ability to conduct electricity and depends on ion concentration, temperature, and ion type/charge.
- Conductivity is measured using a conductivity cell and meter, which applies a voltage to electrodes in contact with the solution.
- The cell constant relates the measured resistance to conductivity and must be regularly calibrated using standard solutions.
- Conductivity increases with temperature so measurements are corrected to 25°C.
- Different ions contribute differently to conductivity based on their charge and concentration. Conductivity can thus estimate total dissolved solids in a solution.