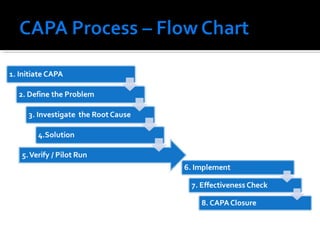
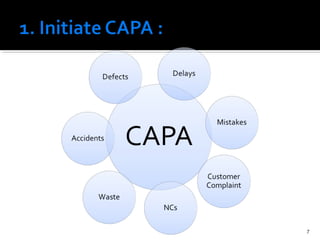
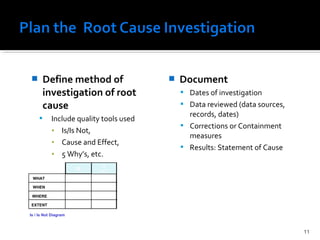
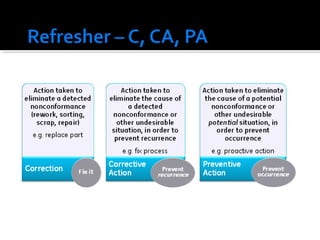
The document discusses the Corrective Action and Preventive Action (CAPA) process. It defines CAPA as a structured way to investigate non-conformities, determine appropriate corrections and actions, and measure their effectiveness. It outlines the key steps in the CAPA process including defining the problem, investigating the root cause, determining corrective and preventive actions, implementing solutions, and measuring effectiveness. The document emphasizes that the goal of CAPA is to eliminate causes of issues in order to prevent recurrence and notes that a mature CAPA system can help continuously improve products, services, and compliance.




![ ISO 9001:2008 : Clause 8.5.2
“The organization shall take action to eliminate the cause of nonconformities in order to prevent recurrence.”
ISO 9001:2015 : Clause 10.2 Nonconformity and corrective action
Clause 10.2.1 When a nonconformity occurs, including any arising from complaints, the organization shall:
a) react to the nonconformity and, as applicable: [Correction]
b) take action to control and correct it;
c) deal with the consequences;
[Nonconformity can be anything deviating from the standard requirements]
b) evaluate the need for action to eliminate the cause(s) of the nonconformity, in order that it does not recur
[Corrective Action] or occur [Preventive Action] elsewhere, by:
a) reviewing and analyzing the nonconformity; [Description of NC]
b) determining the causes of the nonconformity; [RCA of NC]
c) determining if similar non conformities exist, or could potentially occur; [Platforms at Risk]
d) implement any action needed; [Correction]
e) review the effectiveness of any corrective action taken; [Effectiveness Measure]
f) update risks and opportunities determined during planning, if necessary; [Update Risk Documented
Information]
g) Make changes to the quality management system, if necessary. [Update Changes in the Documented
Information]
h) Corrective actions shall be appropriate to the effects of the nonconformities encountered. [Effectiveness
Measure]
Clause 10.2.2 The organization shall retain documented information as evidence of:
a) the nature of the nonconformities and any subsequent actions taken;
b) the results of any corrective action.](https://image.slidesharecdn.com/capa-161227180913/85/Effective-CAPA-Implementation-in-a-Management-System-Praneet-Surti-5-320.jpg)














![28
PRANEET SURTI
Management Auditor l Quality Consultant
[Lead Auditor ISO 9001:2015, B.E Mechanical Engineering]
ANM Strategic & Management Consultants Pvt. Ltd.
Raipur, Chhattisgarh IND
Cell : +91 8109773774 (Personal), +91 7024154549 (Official)
Gmail: praneetsurti20@gmail.com
LinkedIn: http://in.linkedin.com/in/praneetsurti
Twitter: twitter.com/praneet20
About the Trainer](https://image.slidesharecdn.com/capa-161227180913/85/Effective-CAPA-Implementation-in-a-Management-System-Praneet-Surti-28-320.jpg)