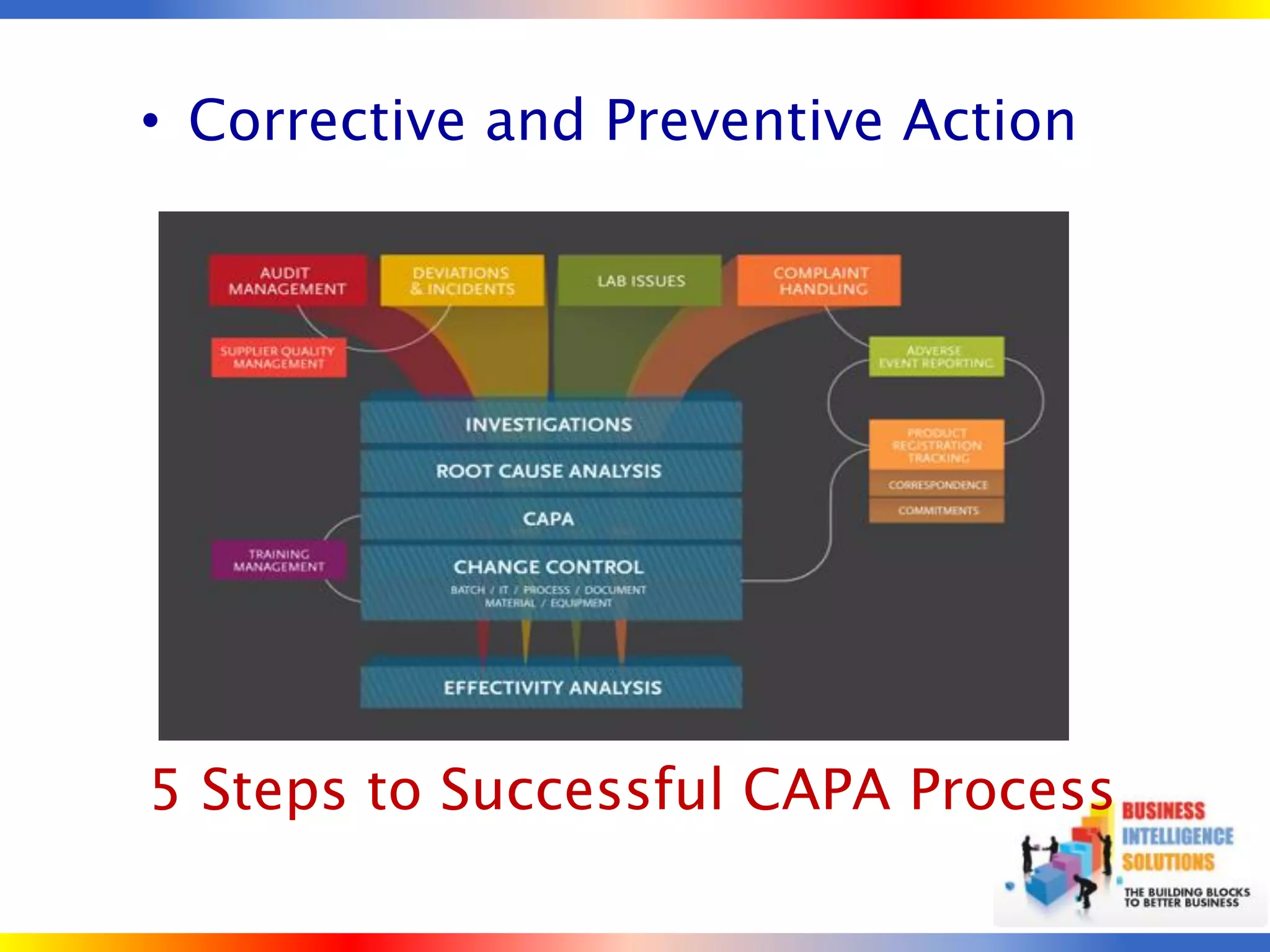
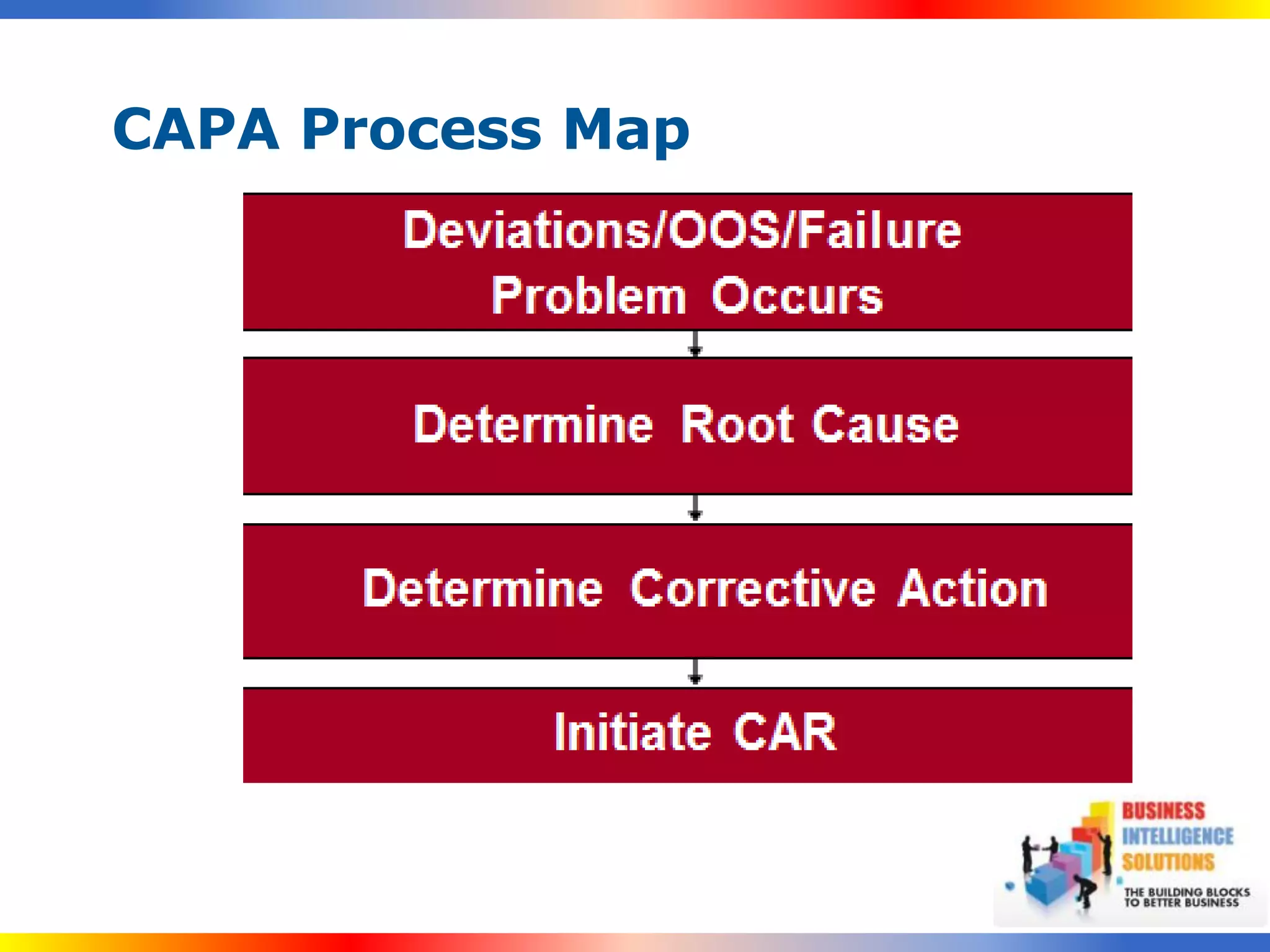
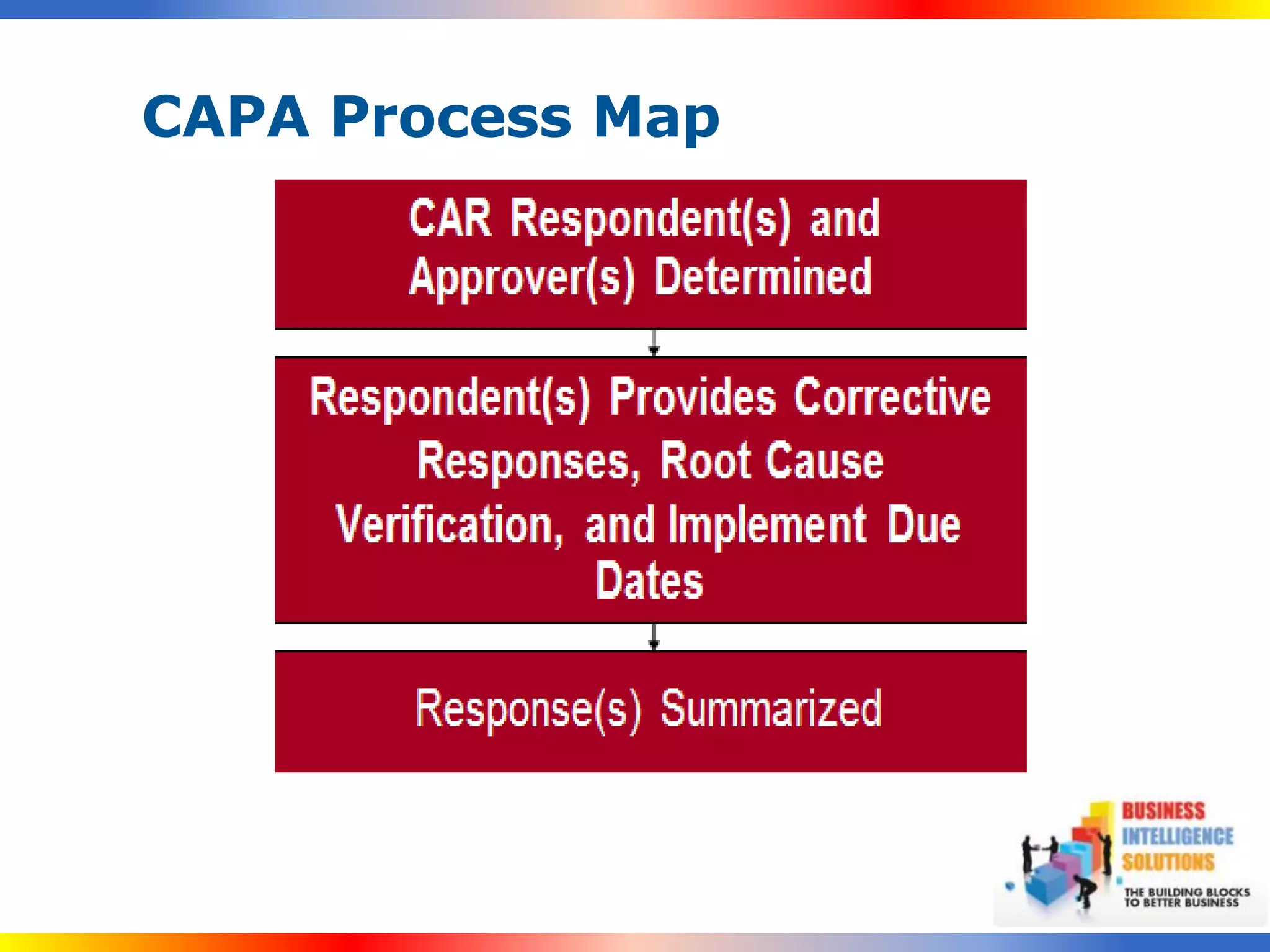
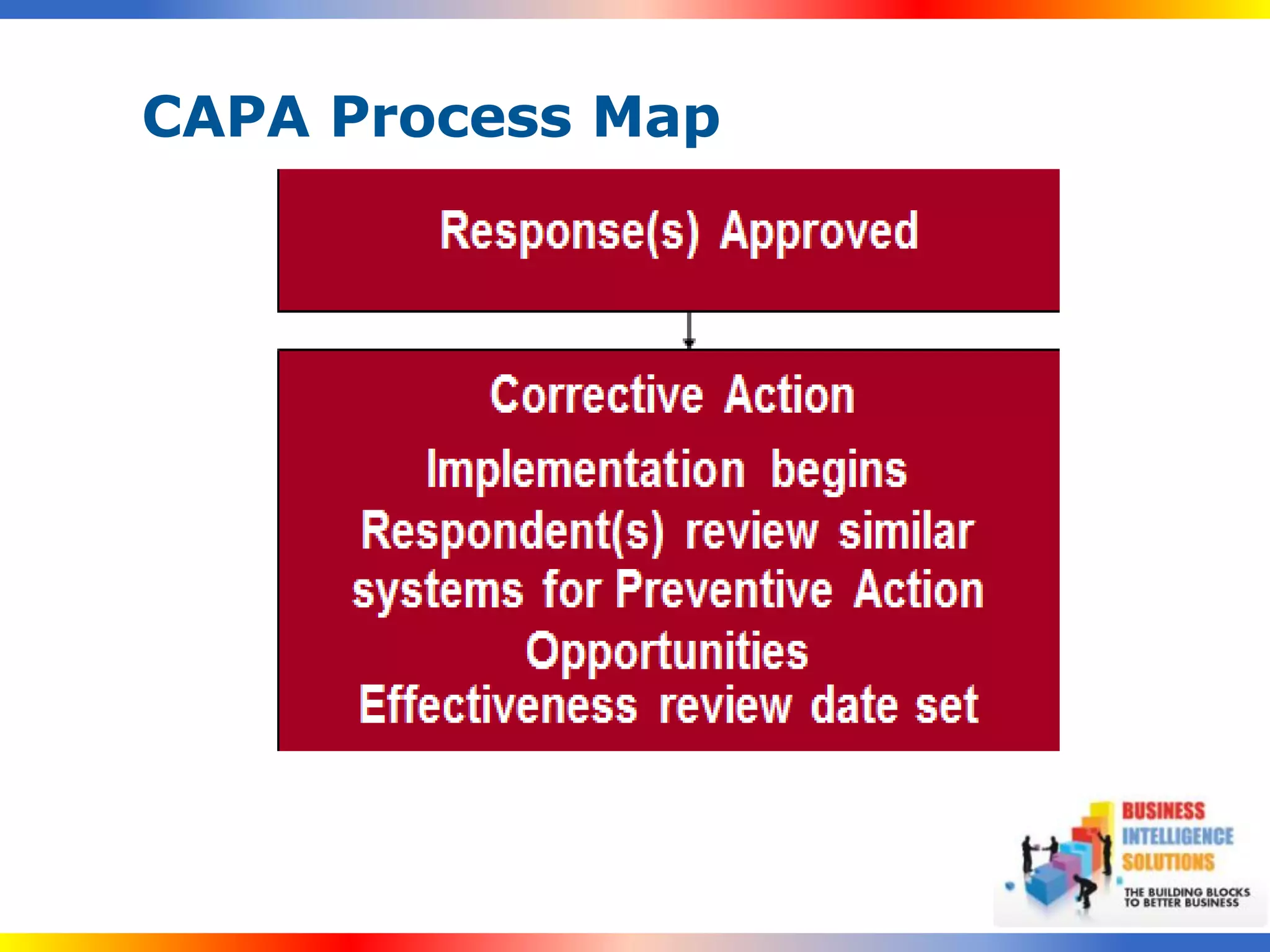
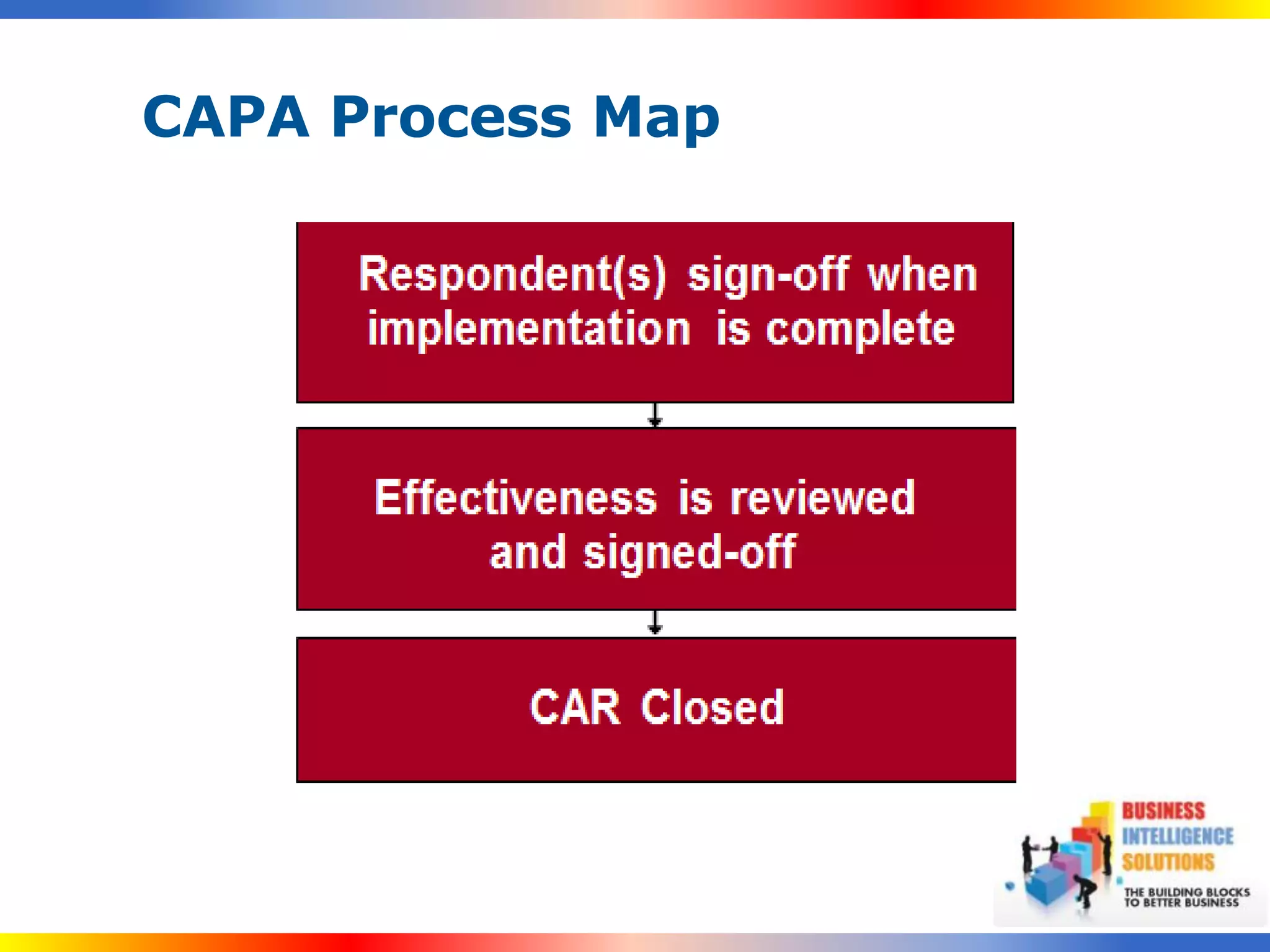
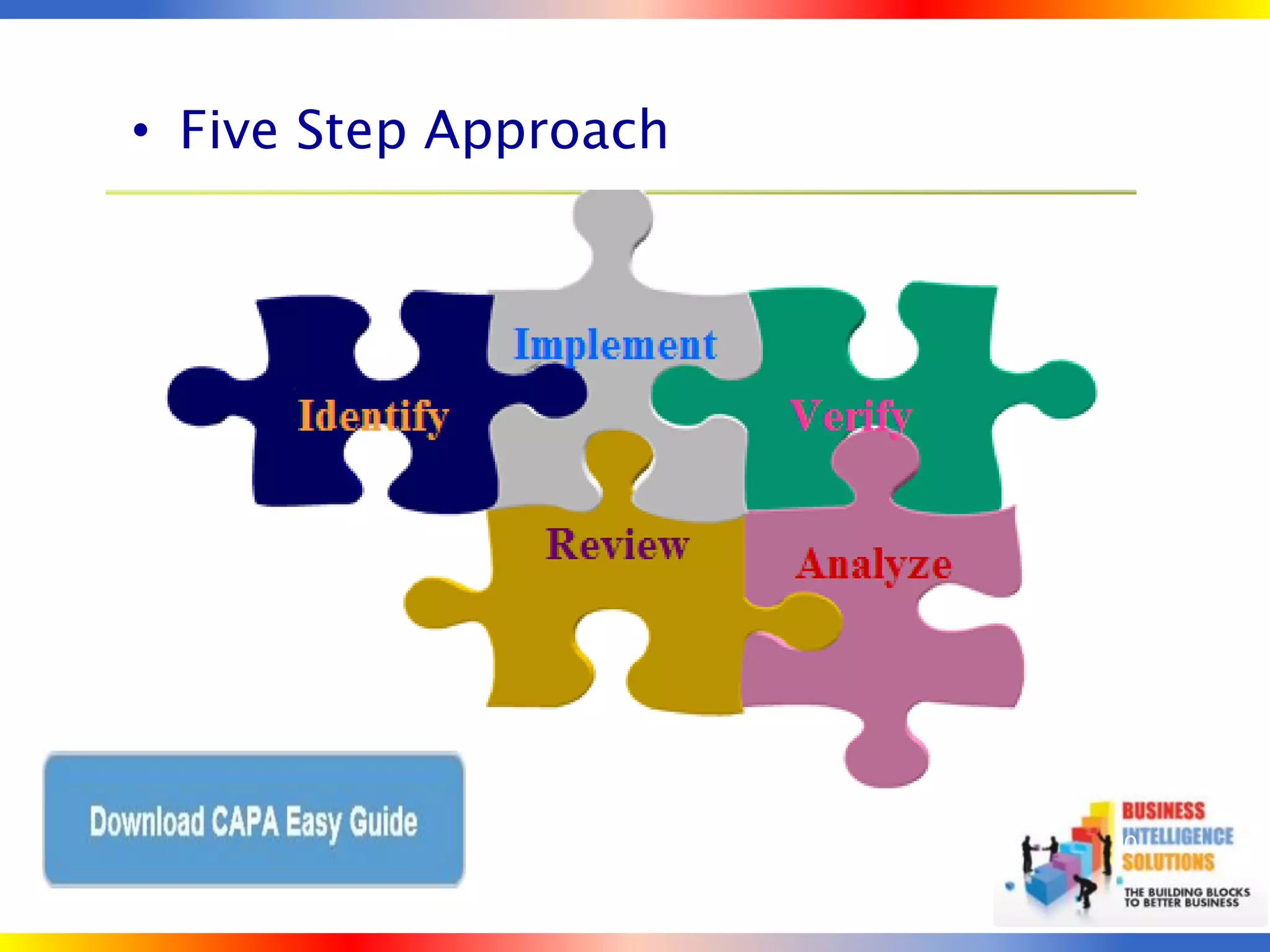
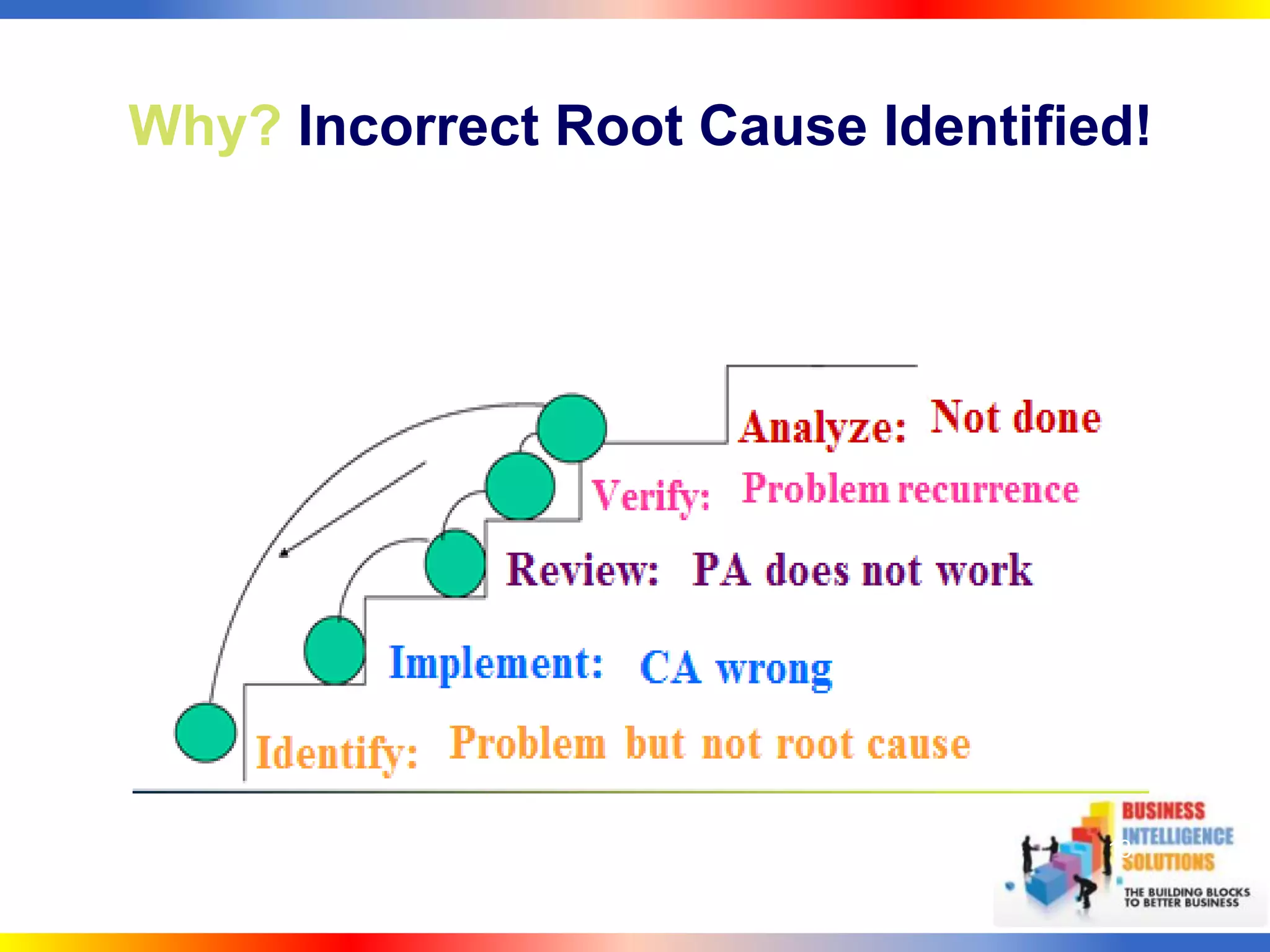
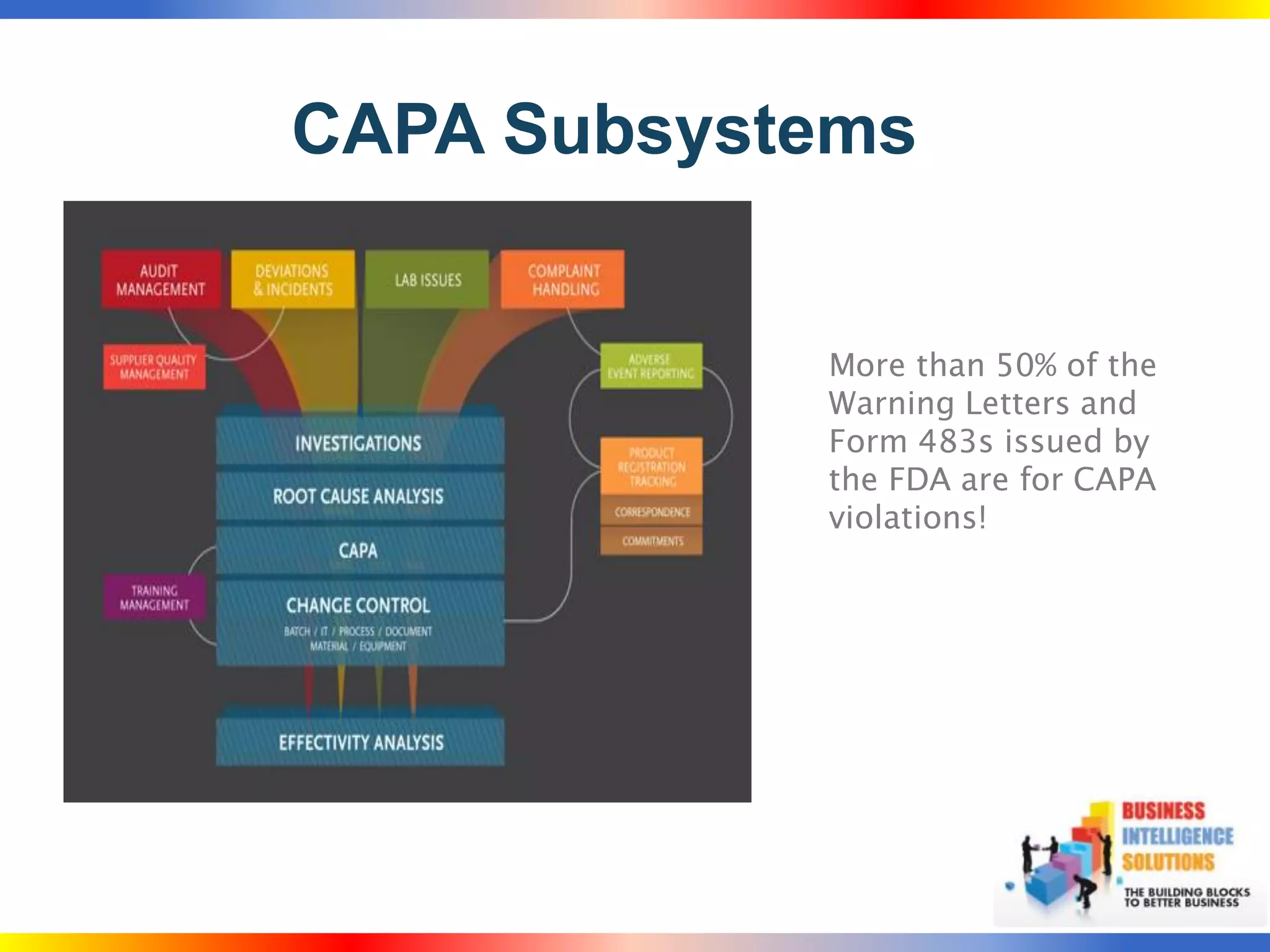
The document outlines a 5-step process for successful Corrective and Preventive Action (CAPA): 1) Identify issues and nonconformances, 2) Implement corrective or preventive actions, 3) Verify that the actions addressed the issues, 4) Review the process for additional improvements, 5) Analyze data for trends. CAPA is important for compliance with Good Manufacturing Practices and focuses on both correcting past issues and preventing future discrepancies.